|
Home Research Publication Glossary Contacts ______________________________________________
|
|
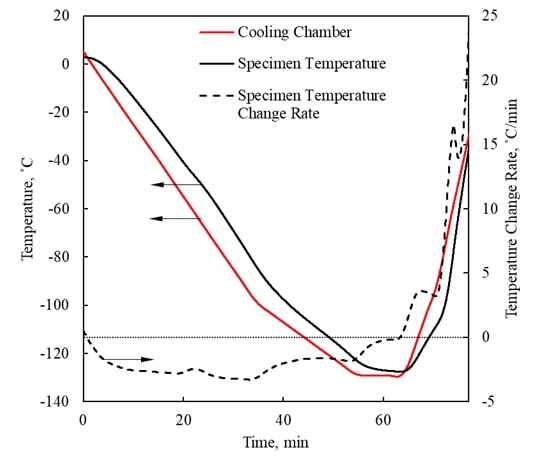
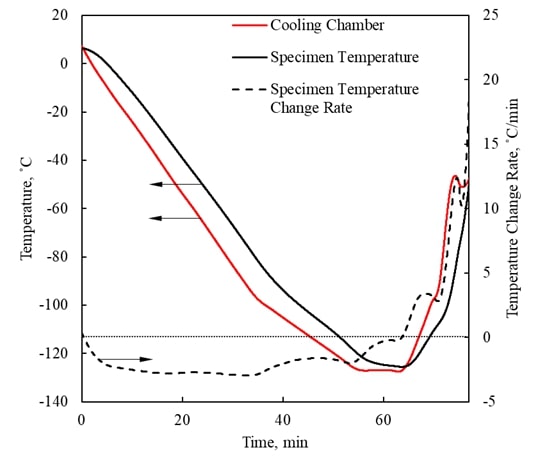
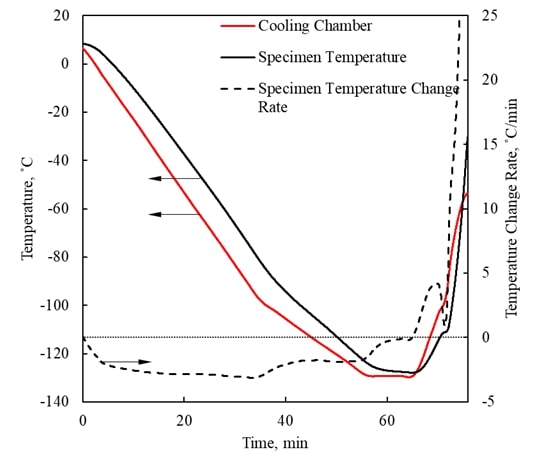
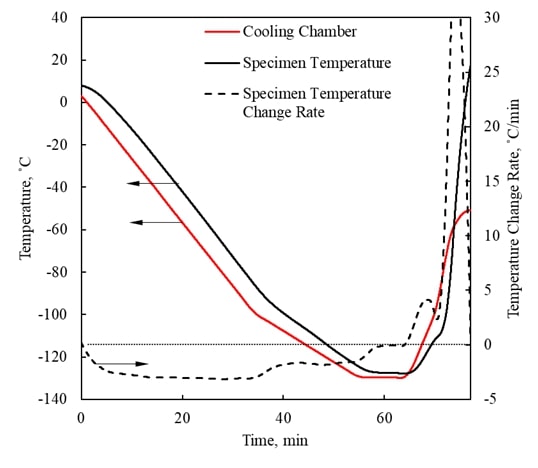
Visualization of Physical Effects during Cryopreservation using DP6 in Combination with Synthetic Ice Modulators (SIMs) The
first cryomacroscope was invented in 2003 and
has been developed ever since at the BTTL. Cryomacroscopy has been demonstrated as an effective
means to visualize physical effects during typical cryopreservation
protocols. Five generations of the cryomacroscope
have been presented over the past 15 years, for various applications, ranging
from measurements of thermophysical properties to
monitoring of large-scale cryopreservation, with the Polarized-Light Scanning Cryomacroscope as the most advanced development thus
far. Presented below are examples of cryomacroscopy
videos from experiments conducted to study Synthetic Ice
Modulators (SIMs) on blood vessels and ovaries (3rd generation cryomacroscopy). |
|
|
|
Pig Femoral
Artery undergoing cryopreservation using 10 ml of DP6 + X1000 + Z1000. |
|
|
|
Pig Femoral
Artery undergoing cryopreservation using 10 ml of DP6 + X1000 + Z1000 +
0.175M Sucrose. |
|
|
|
Pig Femoral
Artery undergoing cryopreservation using 10 ml of DP6 + 0.175M Sucrose. |
|
|
|
Rabbit jugular
vein undergoing cryopreservation using 4 ml of DP6 + 0.5M Sucrose + Unisol. |
|
|
|
Ovary undergoing
cryopreservation protocol using M22 as the CPA. |
|
|
|
Rabbit jugular
vein undergoing cryopreservation in 10 ml of DP6 + 0.175M Sucrose + Unisol. |
|
|
|
Pig Femoral
Artery undergoing cryopreservation using 10 ml of DP6 + 0.175M Sucrose.
Highlighted effects:
|
|
|
|
Pig Femoral
Artery undergoing cryopreservation protocol using 10 ml of DP7 + 0.6M
Sucrose. Highlighted effects: |
|
|
|
Rabbit jugular
vein undergoing cryopreservation using 10 ml of DP6 as CPA + 0.5M Sucrose + Unisol. Highlighted effects: |
|
|
|
Pig Femoral
Artery undergoing cryopreservation using 10 ml of DP6 + 0.6M Sucrose.
Highlighted effects: |
|
|
|
Pig Femoral
Artery undergoing cryopreservation using 10 ml of DP6 + 0.6M Sucrose.
Highlighted effects:
|
|
Selected
Publications: |
||
|
|
Wowk, B.G.,
Fahy, G.M, Ahmedyar,
S., Taylor, M.J., Rabin, Y. (2018): Vitrification tendency and stability
of DP6 vitrification solutions for complex tissue cryopreservation,
Cryobiology, 82:70-77, PubMed, HHS
Public Access, ScienceDirect |
|
|
|
Solanki, P.K.,
Rabin, Y. (2018): Analysis of polarized-light effects in glass-promoting
solutions with applications to cryopreservation and organ banking, PLoS ONE, 13(6): e0199155 PubMed, HHS Public
Access, plos.org |
|
|
|
Ehrlich, L.E., Malen, J.A., Rabin, Y. (2016): Thermal conductivity of
the cryoprotective cocktail DP6 in cryogenic
temperatures, in the presence and absence of synthetic ice modulators,
Cryobiology, 73(2):196-202 PubMed, HHS Public
Access, ScienceDirect |
|
|
|
Feig, J.S.G.,
Solanki, P.K., Eisenberg, D.P., Rabin, Y. (2016): Polarized light scanning
cryomacroscopy, Part II: thermal modeling and analysis of experimental
observations, Cryobiology, 73(2):272-281 PubMed, HHS PublicAccess, ScienceDirect |
|
|
|
Feig, J.S.G.,
Eisenberg, D.P., Rabin, Y. (2016): Polarized light scanning cryomacroscopy,
Part I: Experimental apparatus and observations of vitrification,
Crystallization, and Photoelasticity Effects, Cryobiology, 73(2):261-71 PubMed, HHS Public
Access, ScienceDirect |
|
|
|
Feig, J.S.G.,
Rabin, Y. (2014): The scanning cryomacroscope with applications to
cryopreservation a device prototype, Cryogenics, 62:118128 HHS
Public Access, ScienceDirect |
|
|
|
Feig, J.S.G.,
Rabin, Y. (2013): Integration of polarized light into scanning
cryomacroscopy. CRYO2013-the 50 th
Annual Meeting of the Society for Cryobiology, N. Bethesda, DC (July 28-31),
Cryobiology, 67(3):399-400 ScienceDirect |
|
|
|
Rabin, Y.,
Taylor, M.J., Feig, J.S.G., Baicu, S., Chen, Z.
(2013): A new cryomacroscope device (Type III) for visualization of physical
events in cryopreservation with applications to vitrification and synthetic
ice modulators, Cryobiology 67(3):264-73 PubMed, HHS Public
Access, ScienceDirect |
|
|
|
Rabin, Y., Feig,
J.S.G., Williams, A.C., Lin, C.C., Thaokar, C.
(2012): Cryomacroscopy in 3D: a device prototype for the study of
cryopreservation. ASME 2012 Summer Bioengineering Conference - SBC 2012,
Fajardo, Puerto Rico, USA (June 20-23) ASME
Digital Collection, BTTL
Depository |
|
|
|
Baicu,
S., Taylor, M.J., Chen, Z., Rabin, Y. (2008): Cryopreservation of carotid
artery segments via vitrification subject to marginal thermal conditions:
Correlation of freezing visualization with functional recovery. Cryobiology,
57(1):1-8 PubMed,
HHS Public
Access, ScienceDirect, BTTL
Depository |
|
|
|
Steif,
P.S., Palastro, M.C, Rabin, Y. (2008): Continuum
mechanics analysis of fracture progression in the vitrified cryoprotective agent DP6. ASME Biomechanical Engineering,
130(2):021006 PubMed,
HHS Public
Access, ASME
Digital Collection |
|
|
|
Rabin, Y., Steif, P.S., Hess, K.C., Jimenez-Rios, J.L., Palastro, M.C. (2006): Fracture formation in vitrified
thin films of cryoprotectants. Cryobiology,
53:75-95 PubMed,
HHS Public
Access, ScienceDirect, BTTL
Depository |
|
|
|
Baicu,
S., Taylor, M.J., Chen, Z., Rabin, Y. (2006): Vitrification of carotid artery
segments: An integrated study of thermophysical
events and functional recovery towards scale-up for clinical applications.
Cell Preservation Technology, 4(4):236-244 PubMed, HHS Public
Access, BTTL
Depository |
|
|
|
Rabin, Y., Steif, P.S. (2006): Solid mechanics aspect of
cryobiology, In: Advances in Biopreservation (Baust, J.G., and Baust J.M.,
Eds.), CRC Taylor & Francis, Chap. 13, pp. 359-382 |
|
|
|
Rabin, Y.,
Taylor, M.J., Walsh, J.R., Baicu, S., Steif, P.S. (2005): Cryomacroscopy of vitrification, Part
I: A prototype and experimental observations on the cocktails VS55 and DP6.
Cell Preservation Technology, 3(3):169-183 PubMed, HHS Public
Access, BTTL
Depository |
|
|
|
Steif,
P.S., Palastro, M., Wen, C.R., Baicu,
S., Taylor, M.J., Rabin, Y. (2005): Cryomacroscopy of vitrification, Part II:
Experimental observations and analysis of fracture formation in vitrified
VS55 and DP6. Cell Preservation Technology, 3(3):184-200 PubMed, HHS Public
Access, BTTL
Depository |
|
|
Acknowledgments:
National Heart Lung and Blood Institute (NHLBI) Grant R01HL127618 |
||
|
______________________________________________ |
||