|
Home • Research • Publication • Glossary • Contacts ______________________________________________
|
||||||||||||||||||||||||||||||||||
|
Method A: Prostate Model Reconstruction Cryosurgery applications such as planning, training and the
development of simulation techniques necessitate 3D shapes of the target
region. The current study focuses on reconstructing these shapes for prostate
cryosurgery.
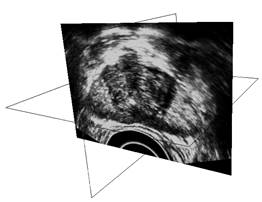
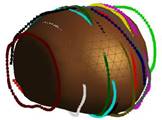
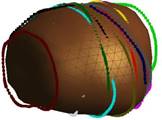
Key steps in prostate model reconstruction: (a) selection of good quality ultrasound cross sections; (b) generation of the prostate contour on each cross section, including illustration of the urethral warmer; (c) generation of polygonal mesh based on cubic Bezier surface interpolation; and, (d) surface rendering of the prostate. Prostate model reconstruction is based on
manual segmentation of ultrasound images. The user selects a series of good
quality ultrasound images of a transverse cross sections, from the apex to
the base of the prostate. On each cross section, the contour of the prostate
is identified by a series of points, having a denser distribution in regions
of higher contour curvature. Next, the points are connected to form a
polyline, which is then approximated as a piecewise cubic Bezier curve. In
order to form a smooth contour, every two adjacent curves are forced to share
the same end point. Furthermore, two control points are added to each curve
so that the slopes of adjacent curves become continuous. This procedure is
repeated for all the selected cross sections, resulting in a series of
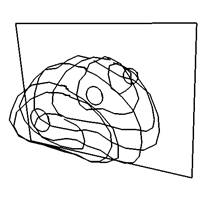
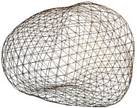
transverse contours, as illustrated in Fig. (b). Once a series of transverse contours is generated, a series of longitudinal cross sections is defined, intersecting perpendicularly with all the transverse contours. Points from all the intersected transverse contours are connected along each longitudinal cross section to form a series of longitudinal polylines, extending from the apex to the base of the prostate. In a method similar to the process of generating of the transverse contours, each longitudinal polyline is approximated as a piecewise cubic Bezier curve, and continuity of position and slope is forced at the end of each curve to ensure the smoothness of the contour. Finally, the surface of the prostate is presented with a polygonal mesh based on bi-cubic Bezier surface interpolation, as illustrated in Fig. (c).
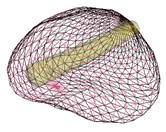
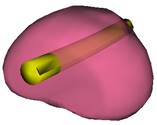
Consistent with current prostate-cryosurgery practice, a urethral warmer is assumed in the prostate, although not originally present in the ultrasound image data. This warmer, embedded in a catheter, is ordinarily inserted into the urethra to keep its temperature above freezing and thereby reduce post-cryosurgery complications. In the current study, the geometry of the urethral warmer is modeled as a 6 mm-diameter tube, having the same centerline as the urethra, as identified from the images. Reconstruction Examples (movies):
Method B:
Interactive Prostate Model Reconstruction
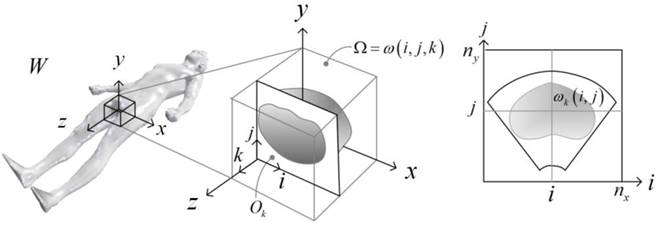
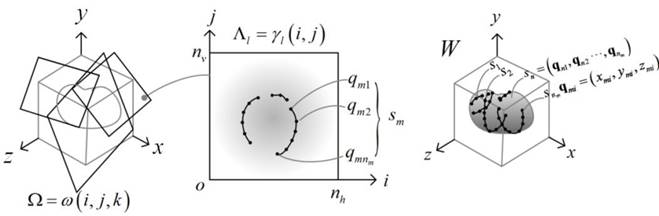
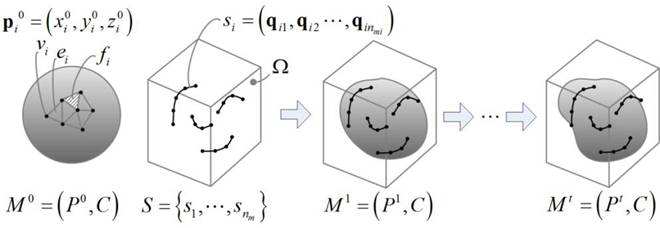
This is a two-step, semi-automated method for reconstructing a
three-dimensional (3D) shape of the prostate from a 3D transrectal ultrasound
(TRUS) image. While the method has been developed for prostate ultrasound
imaging, it can potentially be applicable to any other organ of the body and
other imaging modalities. The proposed method takes as input a 3D TRUS image
and generates a watertight 3D surface model of the prostate. In the first
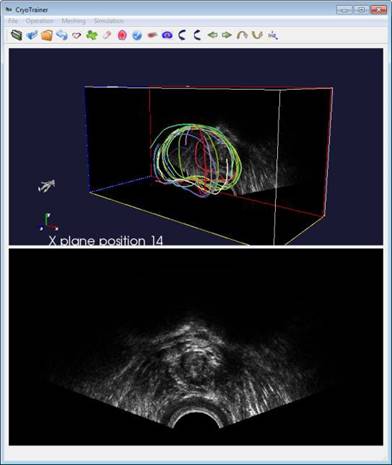
step, the system lets the user visualize and navigate through the input
volumetric image by displaying cross sectional views oriented in arbitrary
directions. The user then draws partial/full contours on selected cross
sectional views. In the second step, the method automatically generates a
watertight 3D surface of the prostate by fitting a deformable spherical
template to the set of user-specified contours. Since the method allows the
user to select the best cross-sectional directions and draw only clearly
recognizable partial or full contours, the user can avoid time-consuming and
inaccurate guesswork on where prostate contours are located. By avoiding the
usage of noisy, incomprehensible portions of the TRUS image, the proposed
method yields more accurate prostate shapes than conventional methods that
demand complete cross-sectional contours selected manually, or automatically
using an image processing tool. Our experiments confirmed that a 3D
watertight surface of the prostate can be generated within five minutes even
from a volumetric image with a high level of speckles and shadow noises.
Reference: •
Furuhata,
T., Song, I., Rabin, Y., Shimada, K. (2014): Interactive prostate shape
reconstruction from 3D TRUS images, Journal of Computational Design and
Engineering, 1(4):272-288 ScienceDirect,
BTTL
Depository
This research has been supported, in part, by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) NIH Grant # 1R01EB003563
|
||||||||||||||||||||||||||||||||||
|
______________________________________________ |
||||||||||||||||||||||||||||||||||