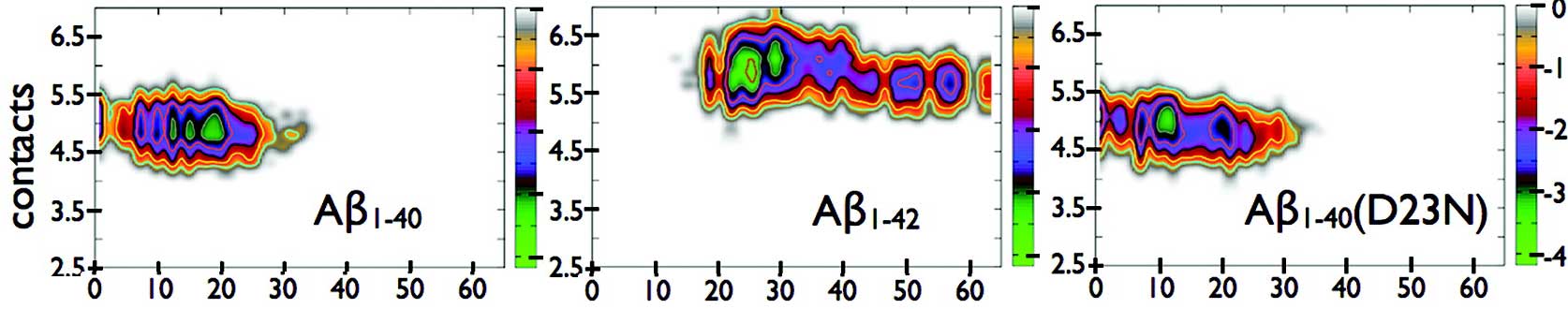
HT-REMD method is also applied to study the pathway of β-amyloid assembly process and the structures of full length Aβ (1-40 and 1-42 and 1-40 (D23N)) (paper number 15 in the CV). In this study, we analyzed in detail the impact of dimerization on the secondary and tertiary structures of three physiologically relevant Amyloid-β alloforms: Aβ1–40, Aβ1–42, and Aβ1–40(D23N). Our simulations clarify the role of Ile41 and Ala42 and D23N on the dimerization of the Aβ peptide.
For Aβ1–42, the two additional hydrophobic residues at the C-terminal, Ile41 and Ala42, have a strong impact on folding. They increase the overall hydrophobic contact and β-strand propensities, and reduce the presence of electrostatic contacts as well as the solvent accessibility of the residues at the CHC and C-terminal. The C-terminal of Aβ1–42 is also involved in more diverse interactions with the other parts of the peptide. Morphologies with globally higher β-strand and hydrophobic contact propensities are favored, as shown by the free energy analysis. The mutation D23N enhances the conformational freedom of the positively charged K28, causing an increase of β-strand propensity at the C-terminal relative to the wild-type Aβ1–40. The presence of β-stranded motifs at the C-terminal such as an intermolecular antiparallel β-sheet could be important to facilitate nucleation. In addition, the free energy landscape of the D23N variant shows that this alloform increases the population of configurations with larger β-strand propensities relative to the wild-type Aβ1–40.
Distinct dimerization for various alloforms
of the Amyloid-beta protein: Aβ1-40, Aβ1-42 and
Aβ1-40(D23N), Sébastien Côté, Rozita Laghaei, Philippe Derreumaux and
Normand Mousseau, J. Phys. Chem. B 116, 4043-4055 (2012).