|
|---|
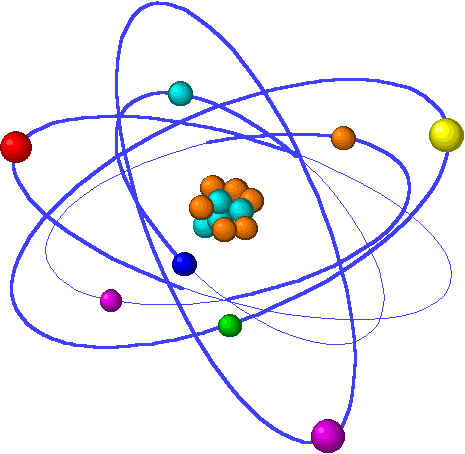
| Atoms are a very important thing in life. Everything is made of atoms. If they didn't exist, nothing would exist except for, well, nothing.
Moving on, an atom has a core called a nucleus made of small spheres called protons and neutrons. All atoms do not have the same amount of protons and
neutrons. Smaller spheres called electrons orbit the nucleus. The number of electrons around an atom is equal to the amount of protons, unless the atom has
taken electrons from another atom (this is because protons are postively charged, and electrons are negatively charged, so they attract each other. Neutrons,
on the other hand, do not have any charges, so they are neutral). Anyways, the reason why we have so many different kinds of elements is because there
are many combinations of protons, neutrons, and electrons that humans have found. Those diffent combinations are the elements that we know
of. So, there you have it: the atom. |
|
| Back to contents |
|---|
Mixtures and Solutions |
|---|
| So, a solution is when a bunch of chemical are mixed together and stay that way. A solution can't be un-mixed easily, like by filtering it or
using machines. An example of a solution is when water and salt mix. However, a mixture is a bunch of elements and compounds that don't mix together
completely. The air is an example of a mixture; it is made of oxygen, nitrogen, and some other elements, but those elements do not share electrons or have a
bond, which means it is not something other than all of its parts. |
|
| Back to contents |
|---|
Elements |
|---|
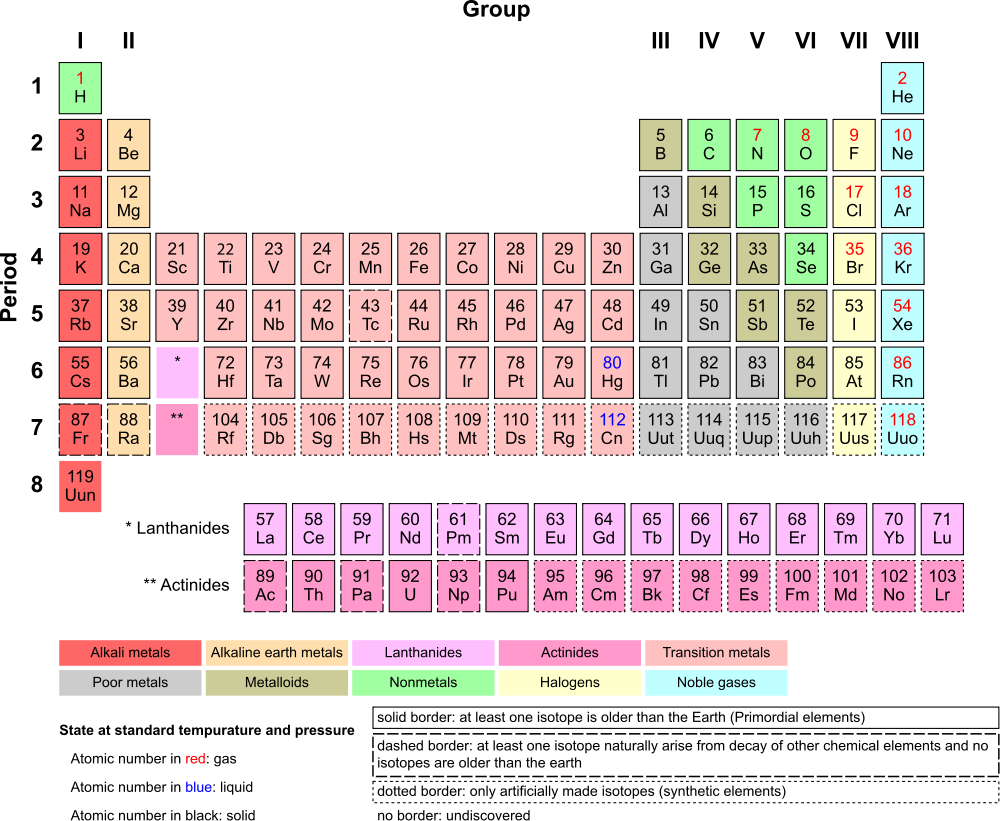
| Elements are the different kinds of atoms that exist which we know of (when I say different kinds, I mean that each element has a different
combo of protons, neutrons, and electrons). The periodic table (see the link for more info) is a record of all of the elements. Currently, there are 118
elements known to us. Each element has its purpose in the world (except for the most rare ones). |
|
| Back to contents |
|---|
Isotopes |
|---|
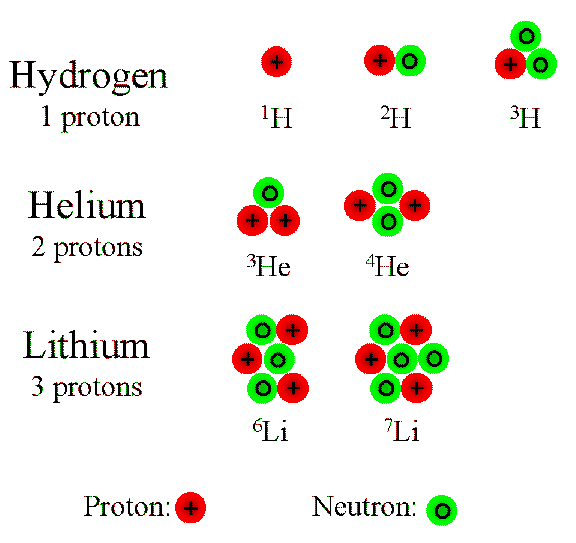
| Isotopes are elements. They just have more or less neutrons than they should have. They exist to separate protons, so they are stable. If the
neutrons were not there, the protons would fly apart because of their positive charges. It's like when you try to make 2 magnets connect with the north
(or south) side only. They just won't get together. So back to isotopes, the reason why an element's atomic mass rarely is an even number is because of their
isotopes. They all are different weights, so chemists use the average of atomic mass on a periodic table. |
|
| Back to contents |
|---|