 Epitaxial Graphene on Silicon Carbide
Epitaxial Graphene on Silicon Carbide
Graphene is the name given to one (or a few) monolayers of hexagonally-arranged carbon
atoms, as pictured below. Within the plane of atoms there are σ-bonds formed from sp2
hybrid orbitals between the carbon atoms, as well as π-bonds formed from pz orbitals
extending out of the plane.
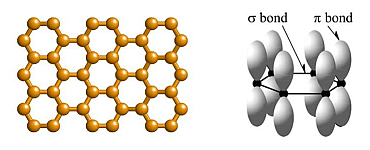
The band of states formed from the pz-orbitals is known as the π-band, and each carbon atom contributes
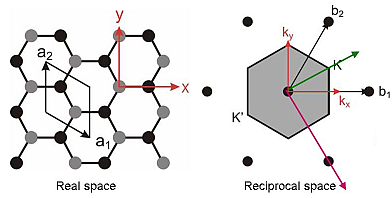
one electron to this band so that it is only half-filled. Additionally, the unit cell of single-monolayer graphene
contains two carbon atoms, which, for certain directions in the plane, form two equidistant lines of atoms. For those
particular directions (towards the K and K' points shown below) the cystal potential does not have a term
at a wavevector value corresponding to the full period of this arrangement. Therefore the upper and lower portions
of the π-band touch at these points (and only these points), which are known as the Dirac points.
The bands thus form a zero bandgap semiconductor (or zero overlap semimetal), with linear dispersion around the
Fermi-energy.
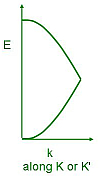
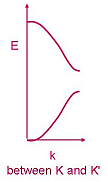
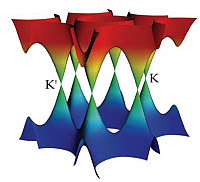
A 3-dimensional representation of the band structure is shown below, taken from M. I. Katsnelson, Materials Today
10, 20 (2007). As a consequence of the linear dispersion around the Dirac points, the transport of carriers
occurs in a unique manner as if the carriers were relativistic particles (since relativistic particles are also
governed by a linear dispersion relation).
Research into the preparation and properties of graphene has been actively pursued
world wide since about 2004. There are two main methods of producing graphene: exfoliation
of monolayer graphene sheets from graphite (i.e. peeling off graphene from graphite), or
preparation of epitaxial graphene on a suitable substrate by deposition of carbon
or by heating of some carbon-containing material. In the latter category, one especially
attractive method is to heat SiC in vacuum at temperatures around 1300°C, during which the
Si atoms preferentially sublimate thereby leaving behind carbon that self-assembles into
graphene.
In Prof. Feenstra's group, this method of high-temperature heating of SiC has been employed since
2007 for the formation of graphene. Samples are heated either of resistive heating (using conductive
SiC) or by the use of a custom-made
graphite heater. The latter
method is suitable for preparation of graphene on semi-insulating SiC, as needed for fabrication
of transistors. Studies of the materials properties of the graphene are carried out using
the techniques of scanning tunneling microscopy, Auger electron spectroscopy, and low-energy
electron microscopy.
 Epitaxial Graphene on Silicon Carbide
Epitaxial Graphene on Silicon Carbide Epitaxial Graphene on Silicon Carbide
Epitaxial Graphene on Silicon Carbide



