| Lecture
#36 |
| Review for Exam IV |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| |
 |
| |
 |
| These next few slides were not gone over in class,
but become an optional discussion of bonding in
transition metal ion complexes as covered in Section 19.7
in the text. It uses pure atomic orbitals instead of
hybrid atomic orbitals, but the concepts of atomic
orbital overlap giving rise to bonding, antibonding, and
non-bonding combinations is as we have seen before. The
figure is what we will attempt to explain, starting
closer to the beginning of its construction for an octahedral
geometry. It contrasts in its starting point
with our use of hybrid orbitals on the transition metal
ion. |
 |
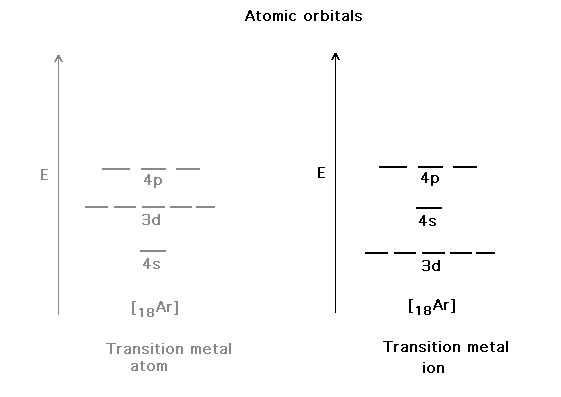
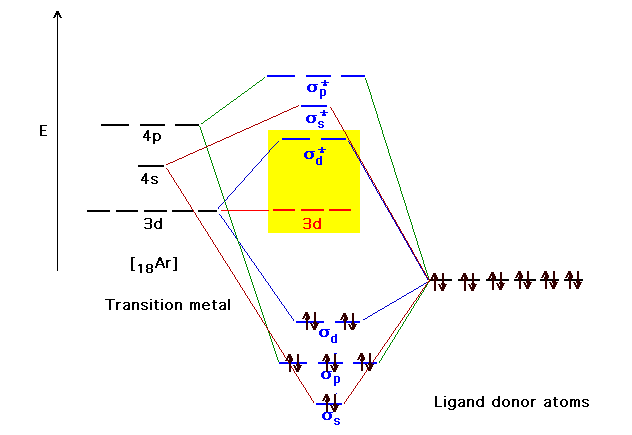
| Atomic orbitals on the metal beyond the closed shell
inner core. Recall that the ion doesn't put its electrons
in any s-valence orbital, but uses d-orbitals instead. |
 |
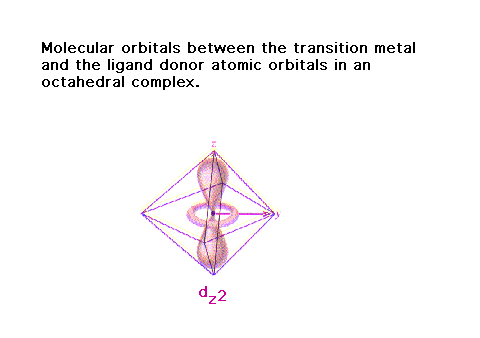
| For ligand orbitals in donor atoms on the x, y, and z
axes, some will overlap properly with the d-orbitals on
the transition metal. |
 |
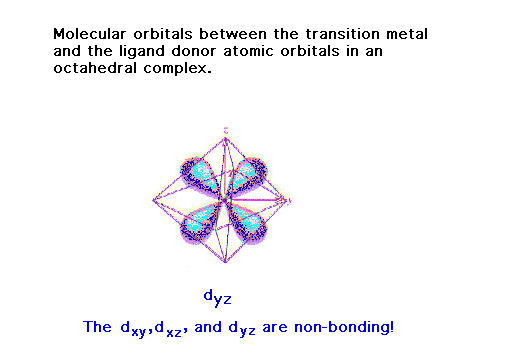
| Some will not. |
 |
| An example where we get a non-bonding orbital between
the donor and the central species. |
 |
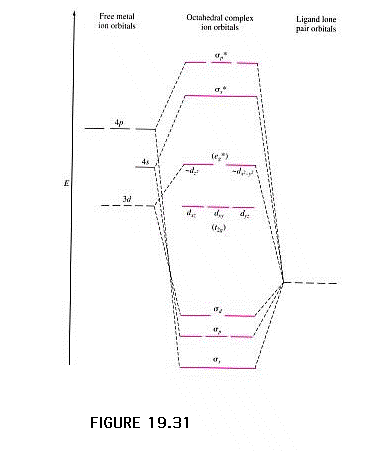
| The molecular orbital energy correlation diagram
showing the resulting bonding, anti-bonding, and
non-bonding combinations. The valence electron pairs are
from each of the six donor atoms in the octahedral
complex. Just above the sigma bonding orbitals are where
the next electrons would go, provided by the transition
metal ion. These are our old friends the t2g
and egbut under different names. |
 |
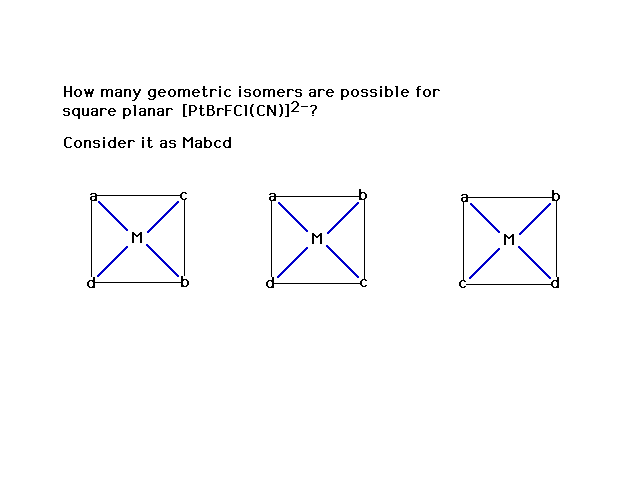
| A practice problem |
 |
| Three isomers. No optical isomers, all geometric. |
 |
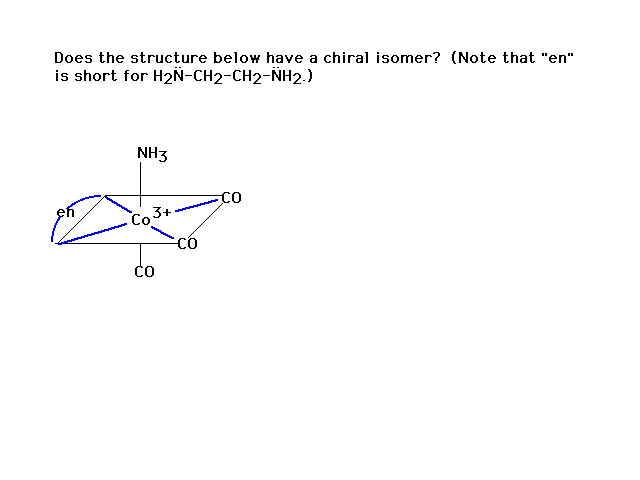
| And another |
 |
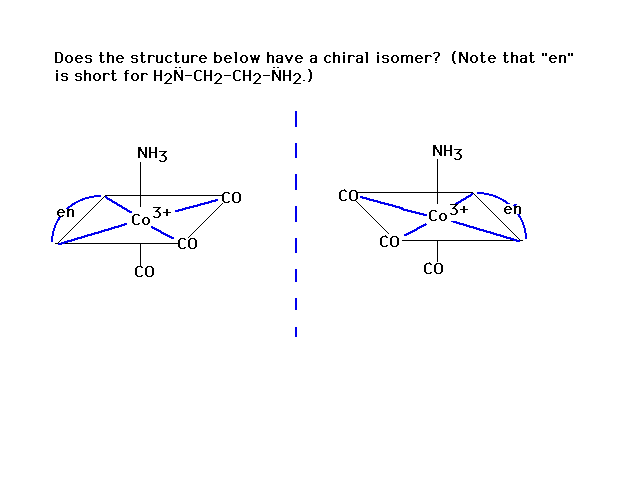
| Rotate the right figure around the verticle axis by
180o and the same molecule results. No isomer. |
 |
| ..on and on we go....(assuming that we have a regular
trigonal prism with equilateral sides) |
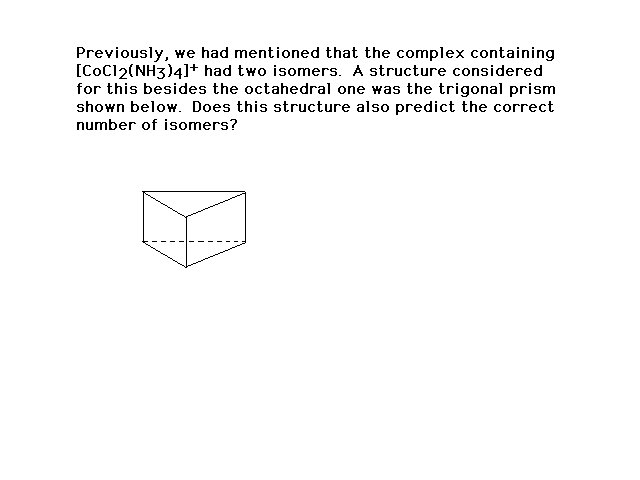 |
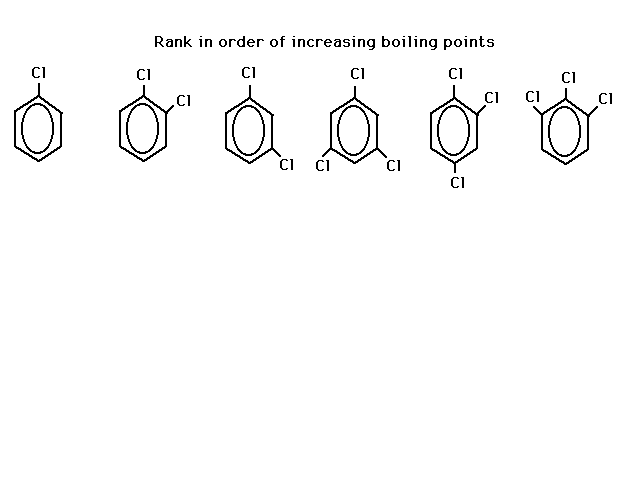
| Rank the following six chlorobenzenes in order of
expected increasing boiling points. |
 |
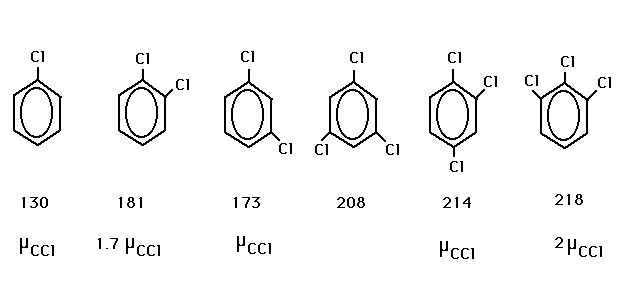
| The relative polarities of the different sets were
actually done in lecture awhile ago. Here are the
polarities and the boiling points. |
 |
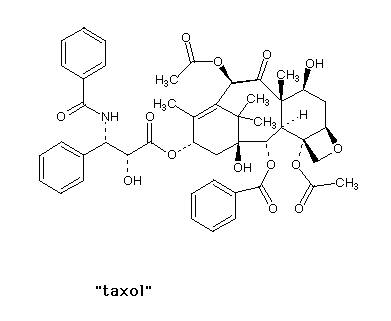
| If each asymmetric carbon gives you two isomers
(enantiomers/chiral isomers), then two such carbons in
the same molecule gives you four possible isomers. How
many isomers of taxol are possible? There will be
twelve 'stereocenters' giving 212 or more than
four thousand possible combinations.
|
 |
| Self-explanatory. (Non-polar, no hydrogen
bonding...size!) |
 |














