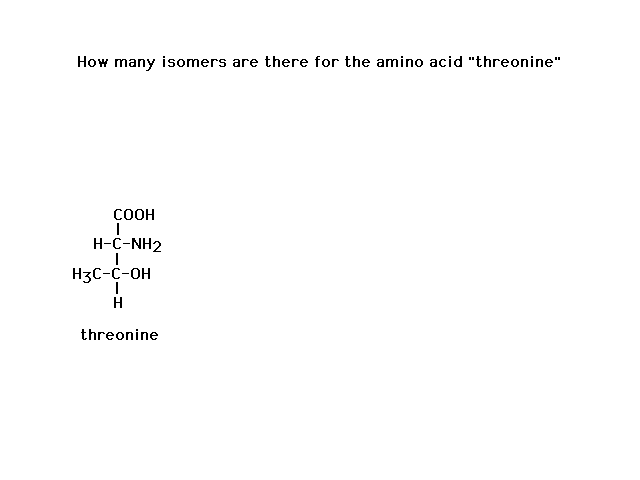| Lecture
#37 |
| Start of review for Exam IV and Exam V |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| The list from which questions will be drawn for Exam
IV. |
 |
| Continuing the list from which questions will be
drawn. |
 |
| |
 |
| Exam V, the "replacement exam" will be
confined to the following topics in addition to those
above for Exam IV, not necessarily covering all of them.
This exam may be taken "risk free". That is, if
it ends up with a lower score than any of Exam I-IV, it
will be ignored. |
 |
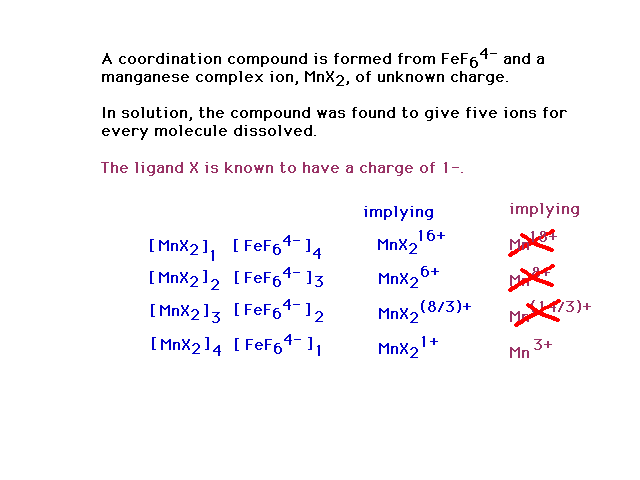
| A review problem for stimulating thought
and discussion. It will
take several steps to work through. It is much too long
and complex to worry about as a possible exam question. |
 |
| |
 |
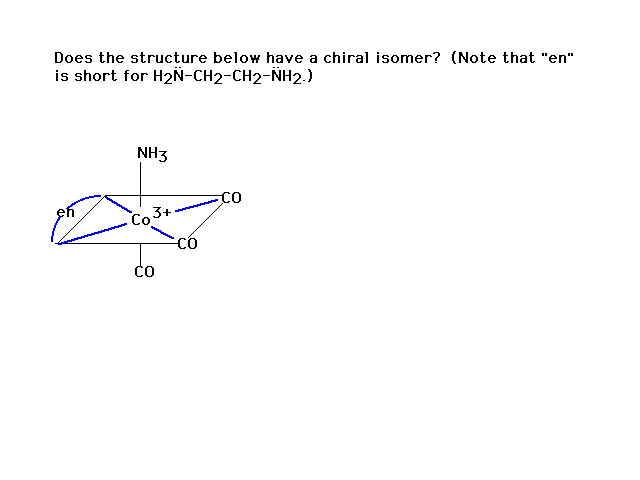
| Another practice problem |
 |
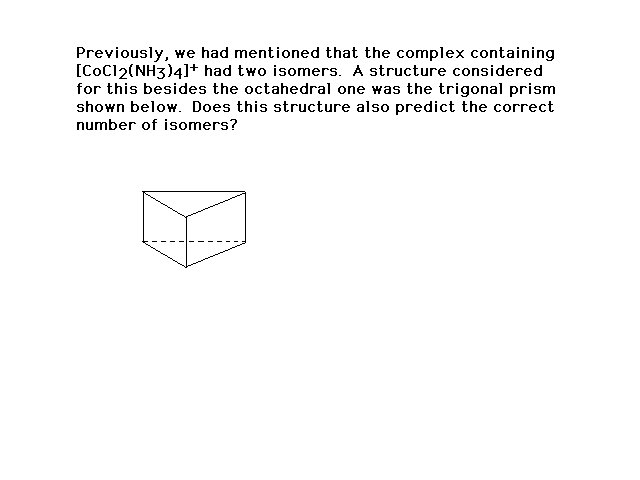
| And another |
 |
| ..on and on we go.... |
 |
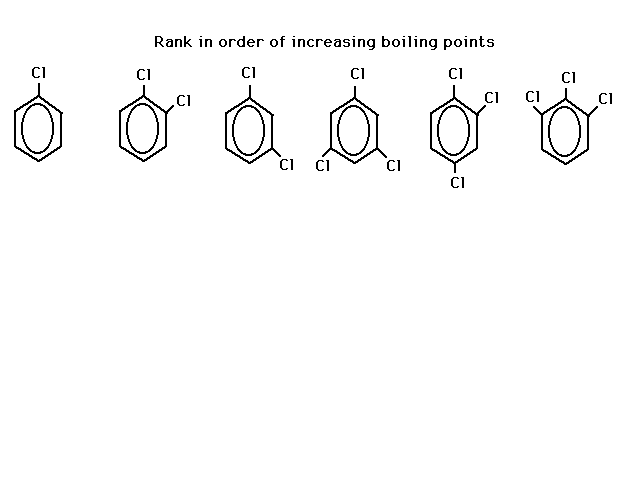
| Rank the following six chlorobenzenes in order of
expected increasing boiling points. |
 |
| That's it! |
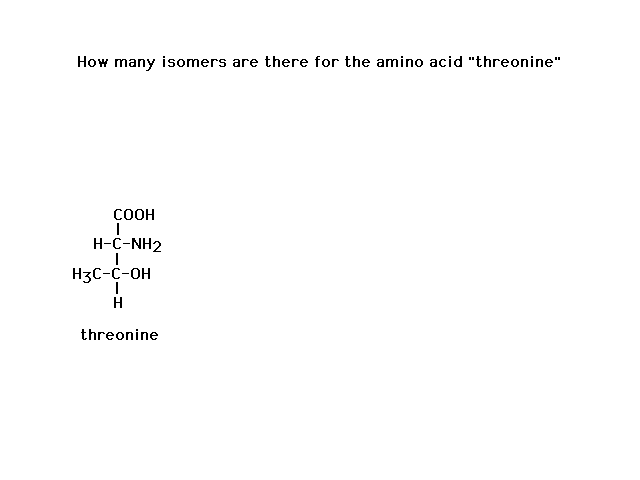 |
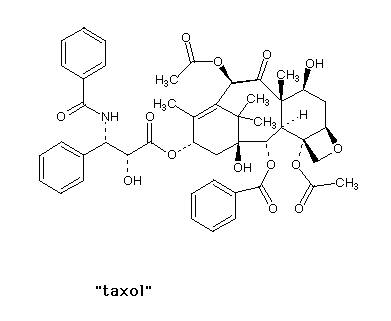
| If each asymmetric carbon gives you two isomers
(enantiomers/chiral isomers), then two such carbons in
the same molecule gives you four possible isomers. How
many isomers of taxol are possible? |
 |
| Self-explanatory. |
 |