| Lecture
#34 |
| Chapter 27 |
CURMUDGEON
GENERAL'S WARNING. These "slides" represent
highlights from lecture and are neither complete nor
meant to replace lecture. It is advised not
to use these as a reliable means to replace missed
lecture material. Do so at risk to healthy academic
performance in 09-105. |
| Lecture Outline |
Proteins and Amino acids
- Amino acids
- alpha amino carboxylic acids
- zwitterions
- side groups (polar vs nonpolar)
- peptide bond
polypeptides and proteins
primary structure
combinations
secondary structure
disulfide links
hydrogen bonds
peptide link pi-bond character
tertiary structure
|
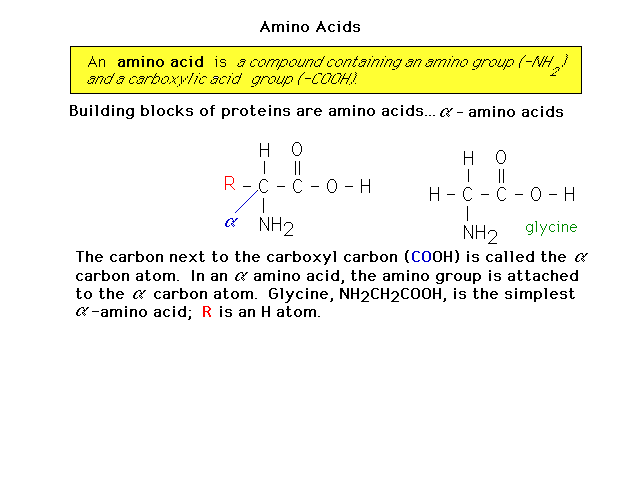
| Proteins are made of amino acids. Here is an
introduction to amino acid structure. |
 |
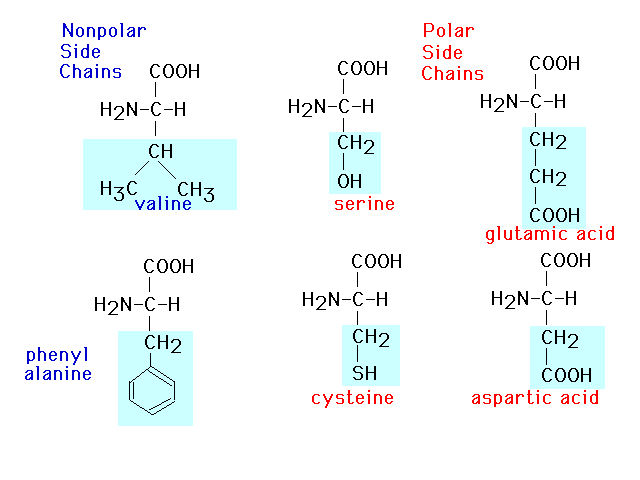
| This presents six of twenty amino acids in
which the polarity of the side groups is called attention
to. |
 |
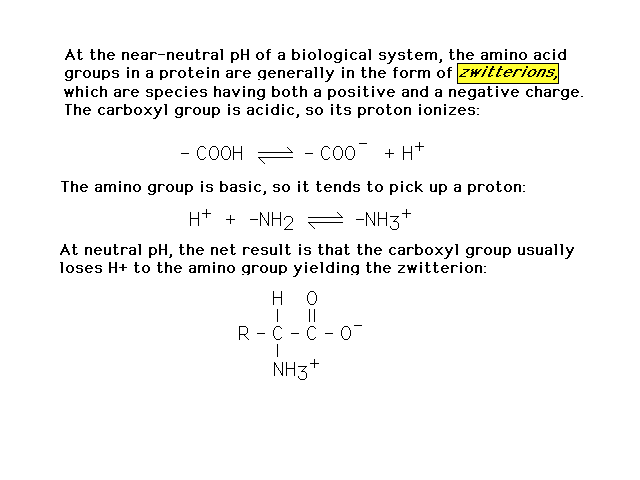
| At pH around 7, an amino acid actually exists
as a structural isomer called a zwitterion, shown here. |
 |
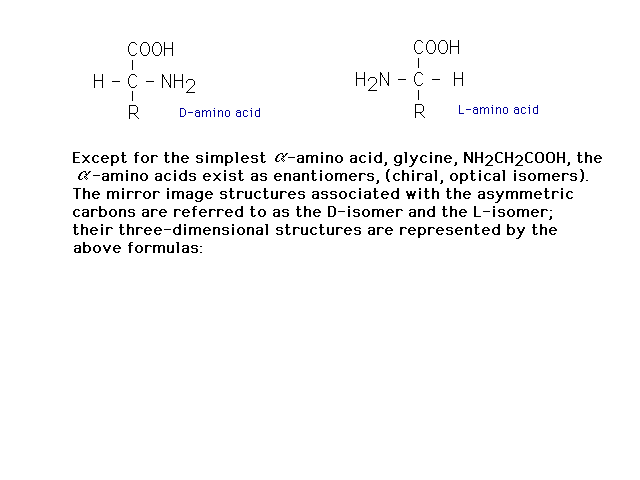
| With the exception of glycine, amino acids are
chiral, having tetrahedral carbons with four different
groups present. |
 |
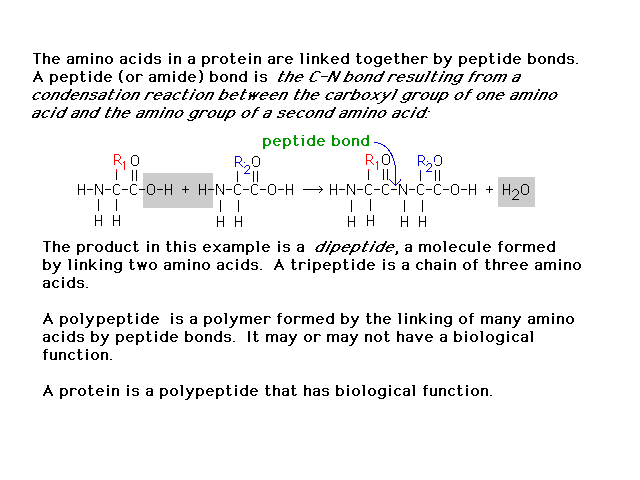
| In building proteins from amino acids, the
links between amino acids are "peptide bonds"
described here. |
 |
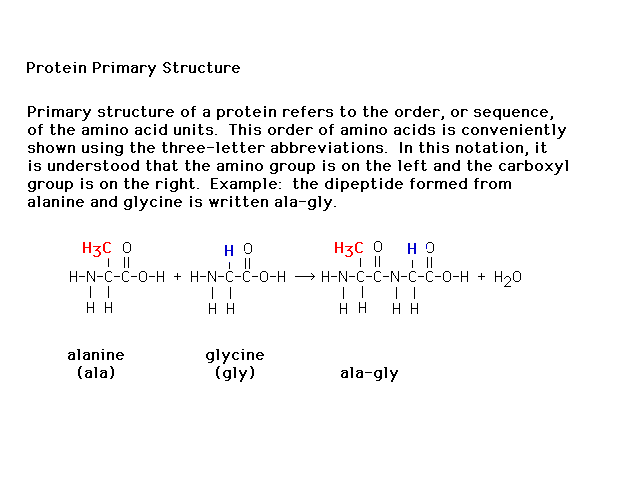
| The sequence of amino acids in a polypeptide
(protein) is referred to as the primary structure. |
 |
| The number of variations of amino acids in
polypeptides is enormous. For 5 different amino acids in
a pentapeptide, thre are 120 possible different
structures. |
 |
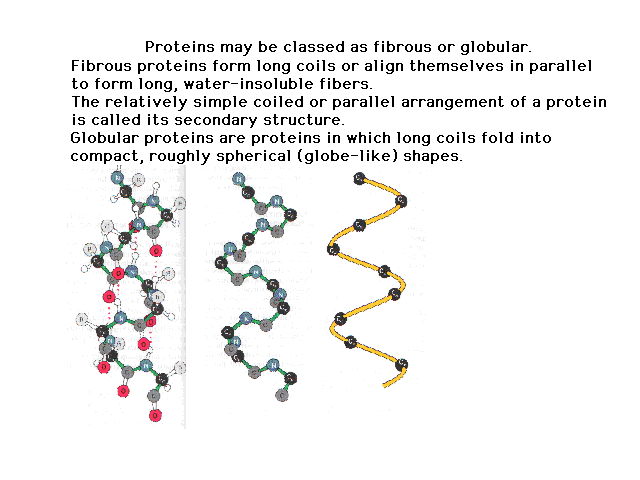
| The sequence defines the primary structure. This
coils up due to properties of the peptide bond and
internal hydrogen bonding to give a secondary structure.
The figure on the right shows the origin of the internal
hydrogen bonding. As we progress to figures on the left,
less and less detail is shown. |
 |
| The coiled secondary structure can further fold into
a globular, tangled geometry defining its tertiary
structure. A familiar example of tertiary structure is
the messy result of coiled extension lines folding up as
shown here. |
 |
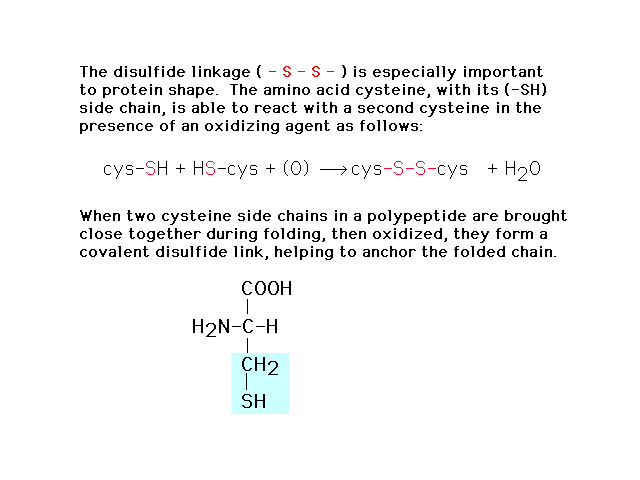
| In forming the three dimensional structure from
the linear (primary) sequence, the S-S disulfide bond
plays a crucial role cross linking sections together. |
 |
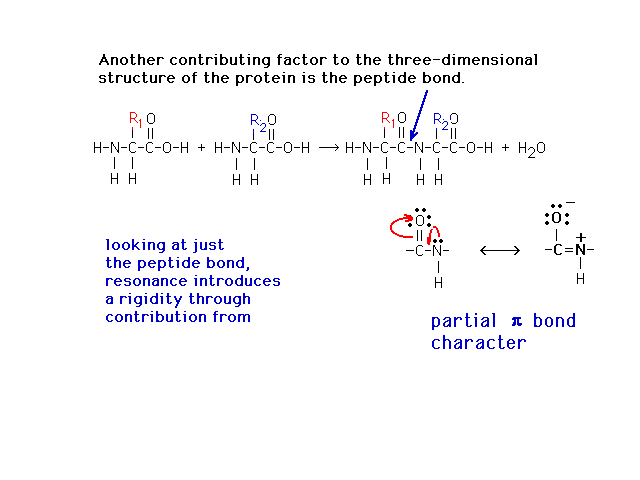
| The peptide bond, which has a Lewis structure
implying looseness associate with a single bond, is
actually quite restricted due to partial double bond
character from a contributing (minor) resonant Lewis
structure as shown. |
 |
| Amino acids are oxyacids in which the -OH group is
bound to a carbon. That carbon is also double-bonded to a
=O and single-bonded to an "amino"
(ammonia-like) -NH2 group.Amino acids are
connected by peptide bonds to form polypeptides. As soon
as you have a number of amino acids joined, the
combinatoric possibilities become staggering. |
 |
| How many different arrangements of 300 amino acids
are possible? |
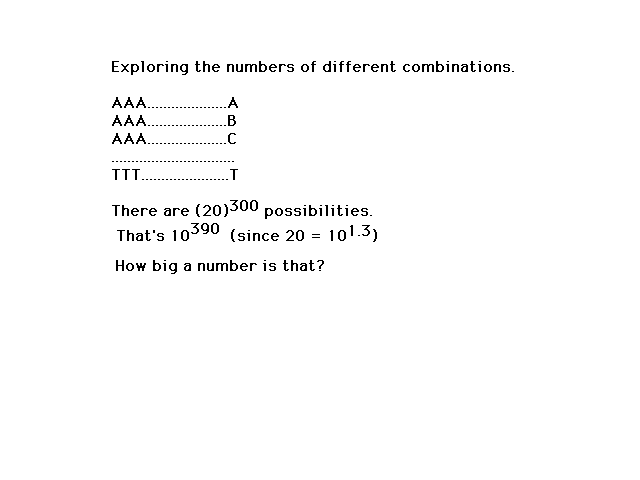 |
| The number of different possibilities is
large...indeed! The universe isn't big enough to be
packed (I mean packed) with just one of
each kind. |
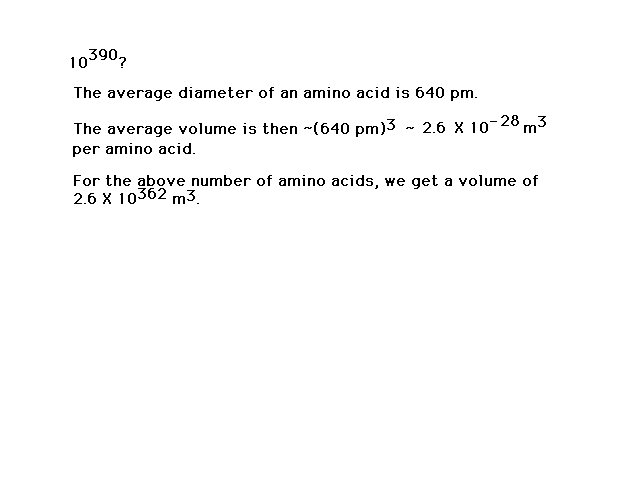 |
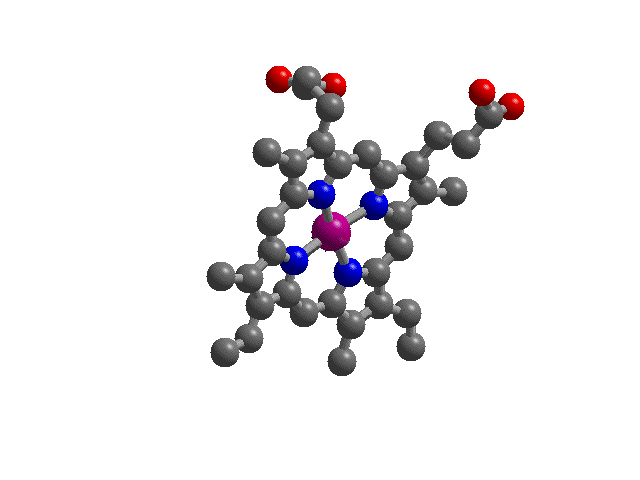
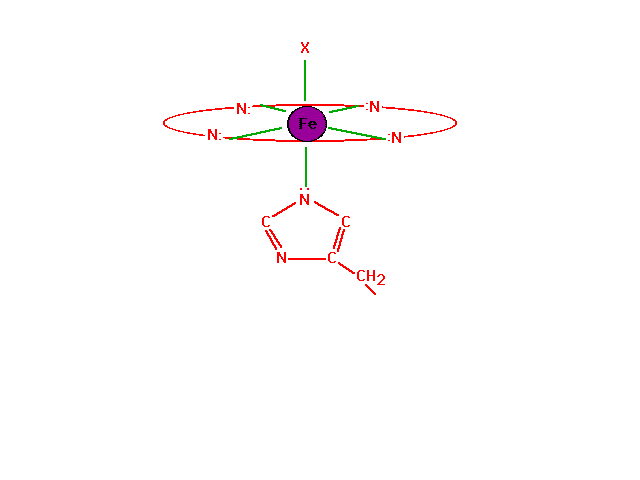
| Hemoglobin: "Heme",
the complex iron ion molecule that runs the essential
chemistry of oxygen transport by blood. In blood, four
hemes are part of a large protein structure called
"hemoglobin". Shown here is "heme"
with the divalent iron ion in purple at its center and
the four (blue) nitrogen donor atoms as part of the heme
ring coordinating ligand.
|
 |
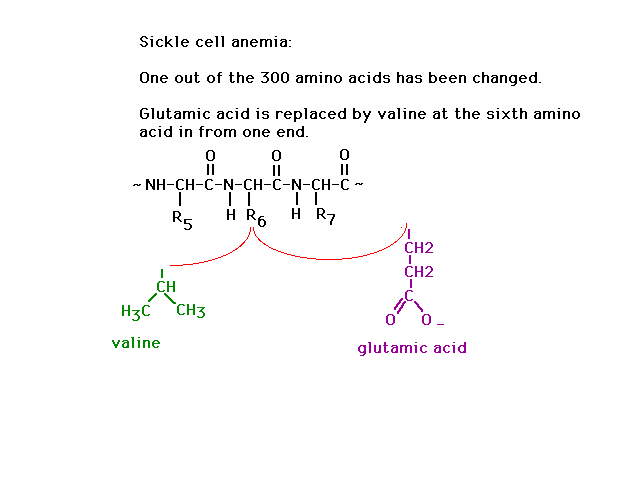
| The genetic disease sickle cell anemia arises when
just one of the amino acids is coded incorrectly:
Glutamic acid is replaced by valine at side group
position number 6 out of 300. |
 |
| Normal red blood cells are smooth and rounded. In
sickled cells, the hemoglobins clump together because of
enhanced intermolecular attractions when the mutually
repelling negatively charged glutamic acid group on each
molecule within a cell is replaced by the neutral valine. |
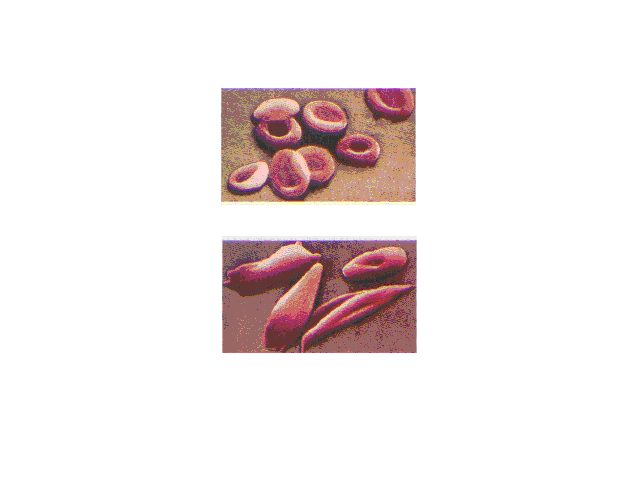 |
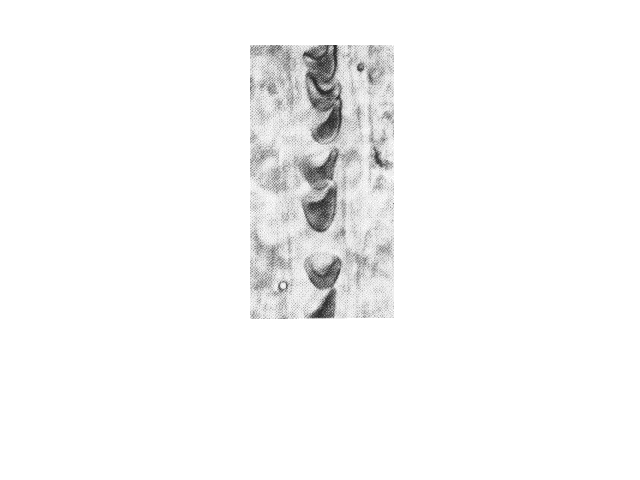
| Sickled cells flowing in a blood capillary. Cell
shape influences the ease of blood flow through the
narrow channels. |
 |
| The chemistry of oxygen in hemoglobin starts with a
look at the Fe2+ at the heme site. |
 |