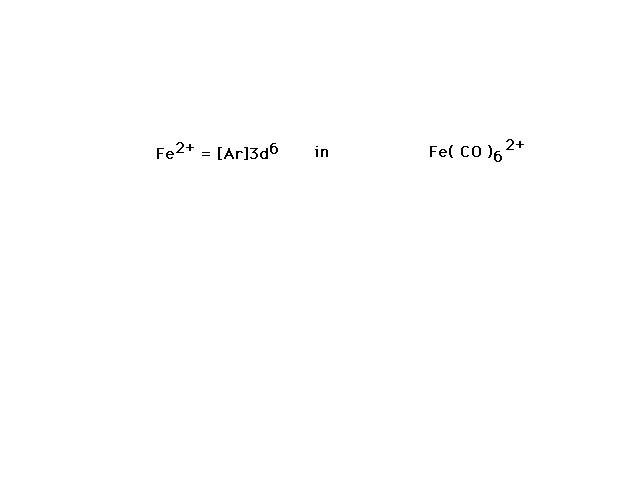| Lecture
#33 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Transition Metal Complexes
Crystal Field theory
Tetrahedral geometry
Hybrid orbitals and bonding
|
| Leaving the discussion of coordination number = 6
(octahedral geometries) and proceeding to coordination
number four. |
 |
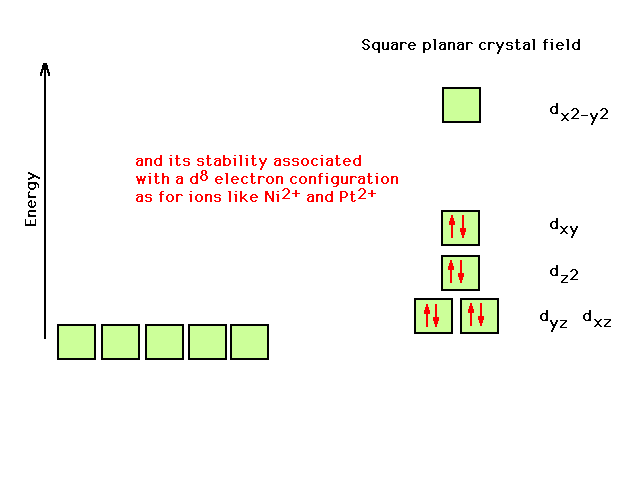
| Now it becomes clear that the square planar geometry
correlates with the observation of extra stability of a
transition metal comlex ion with a d8
configuration on the central atom |
 |
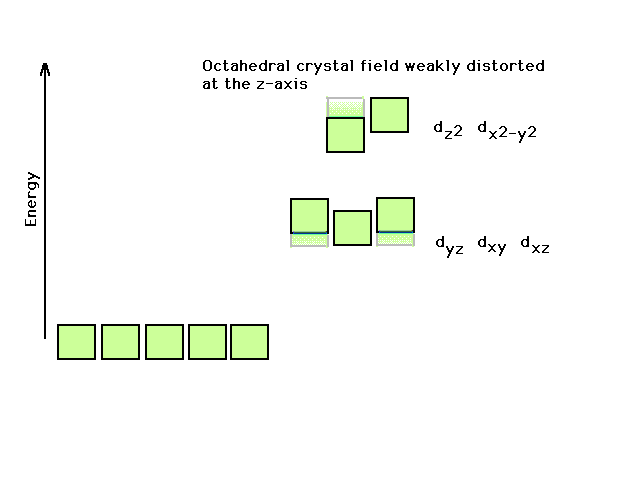
| Returning to an octahedral geometry, we look at the
effect of having a weak ligand plus five stronger
ligands. The weak ligand is assumed to be on the z-axis
which changes the stability of the transition metal ion
valence orbitals directed that way |
 |
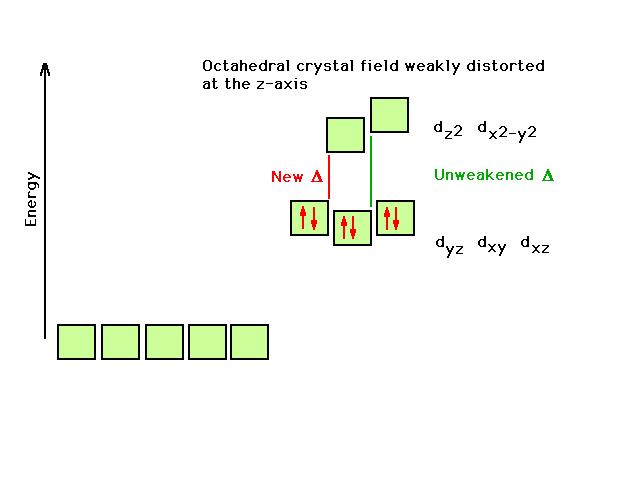
| Co3+ transition metal complex ions with
one of the ligands varying shifts the wavelength of light
absorbed to longer wavelengths as that one ligand becomes
"weaker" in the field it produces. Transition
moves "easiest-to-move electron" up to next
available empty orbital. |
 |
| Measured optical properties (colors) of Co3+
transition metal complex ions. What do you estimate the
color of the last complex to be? This last complex is
meant to be just the trans geometric isomer. |
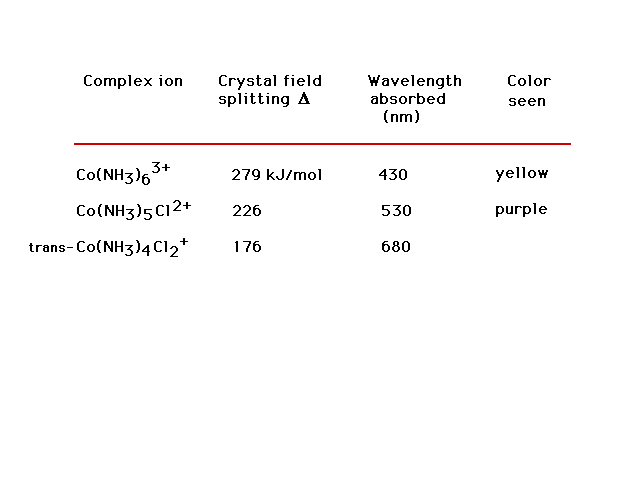 |
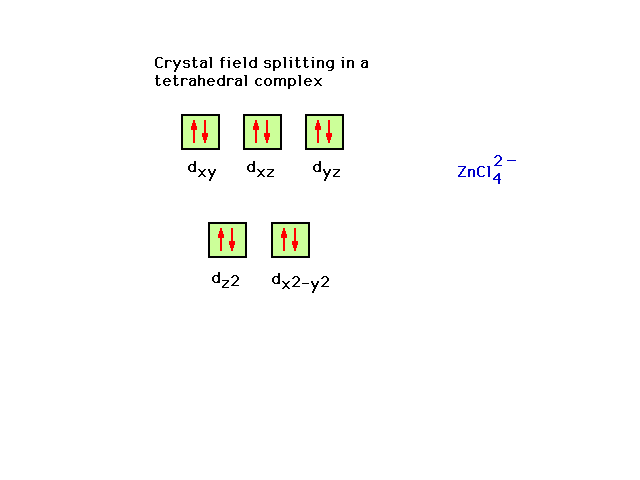
| The tetrahedral crystal field geometry (without
demonstrating how to generate the result) turns out to be
the exact inverse orbital order of that seen in the
octahedral geometry. |
 |
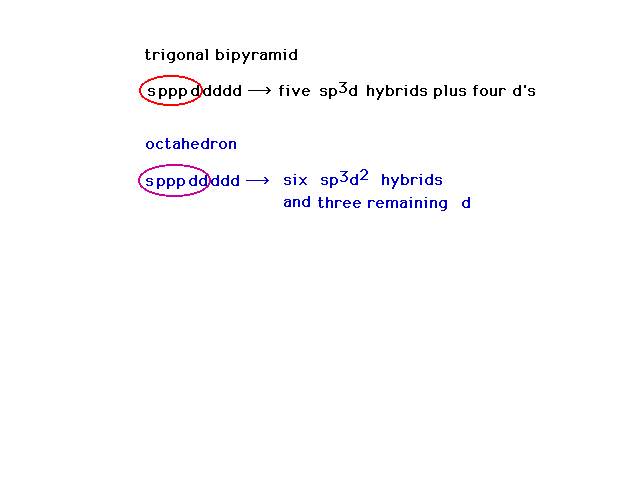
| Crystal field theory told us nothing about the actual
bonds. To begin our discussion of bonding in transition
metal complexes, we return to consideration of hybrid
atomic orbitals. Here is a review of hybrid atomic
orbitals for atoms which can have expanded octets as
would be the case for our transition metal ions. |
 |
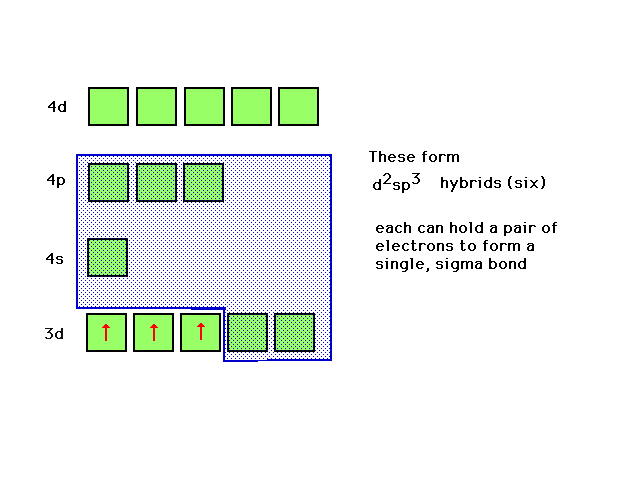
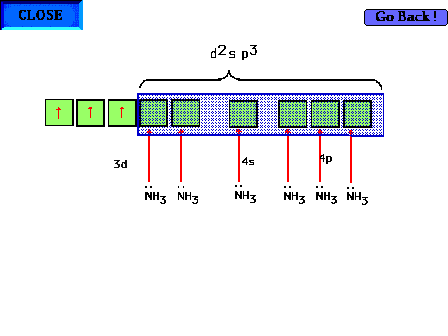
| The highest occupied atomic orbital in Cr3+
and the lowest unoccupied orbitals are shown. The d2sp3
hybrids can be constructed from appropriate choices here. |
 |
| A review of d2sp3 hybrid atomic
orbitals |
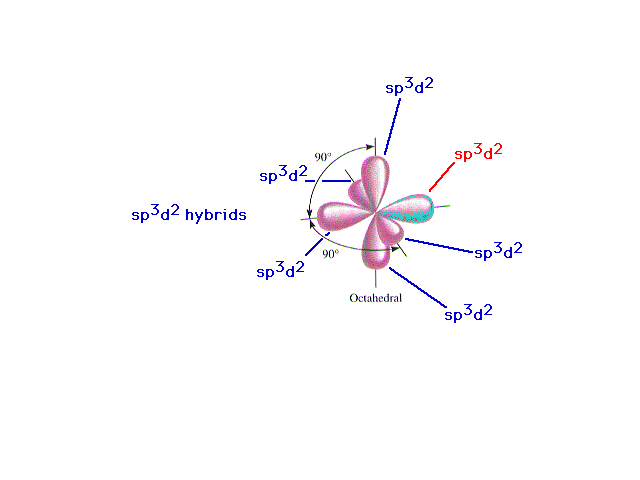 |
| The ammonia ligands bind to the transition metal ion.
In theory, the bond forms in the region of overlap
between the transition metal ion's hybrid atomic d2sp3
orbital and the ligand's hybrid sp3 atomic
orbital. Two electrons go here, both from the ligand, to
form a sigma bond. |
 |
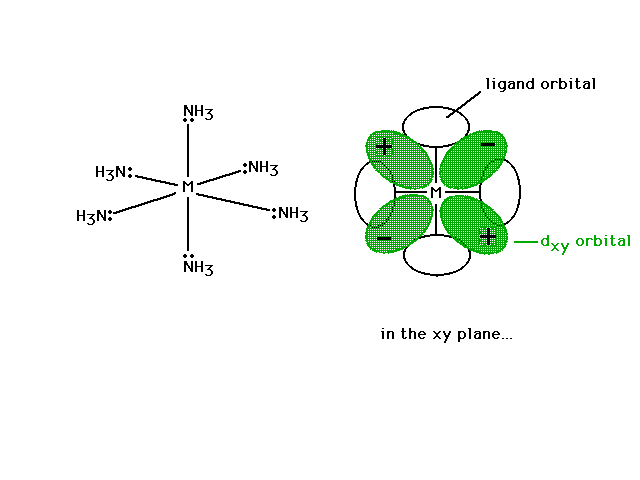
| Looking in just the xy plane where the sigma bonds to
four NH3's are located as is the dxy
non-bonding orbital. The ligand atomic orbital which
would overlap with the transition metal hybrid orbital (a
non-bonding interaction) is shown on the x- and y-axes. |
 |
| Fe(H2O)62+ is our
next example. |
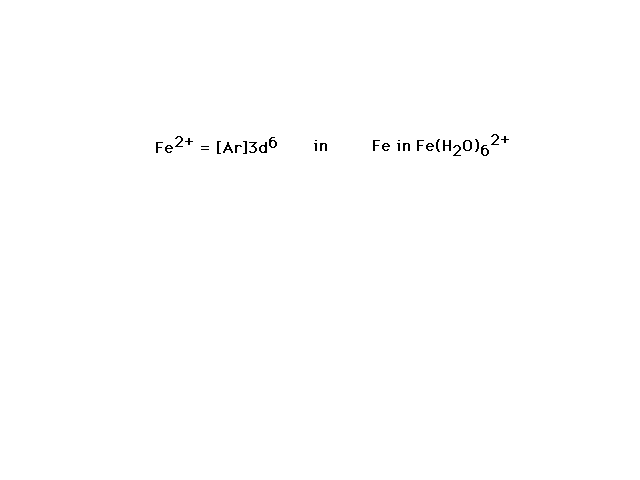 |
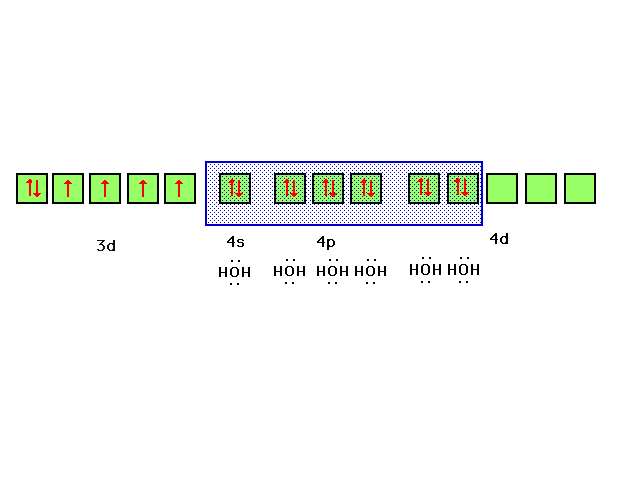
| If we allow iron's six valence electrons to occupy
the 3d orbital as indicated, each water will contribute a
lone pair of electrons to a bond between its sp3
orbital and iron's d2sp3 orbital
formed as indicated |
 |
| Fe(CO)62+ is our next
illustration. Since CO is a strong field ligand, we
should not be surprised to see a low-spin complex ion
electron configuration show up by necessity. |
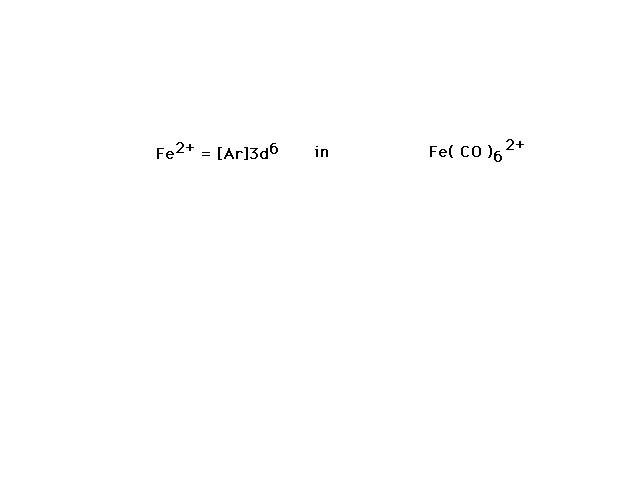 |
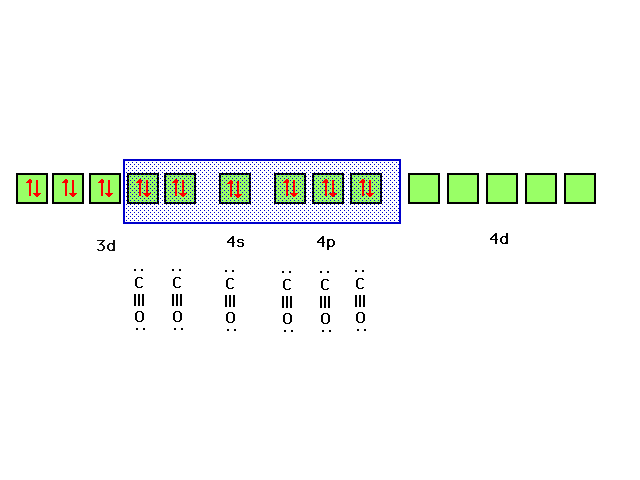
| Bonding of the CO ligands to the d2sp3
hybrid orbitals chosen as illustrated is consistent with
the complex ion being diamagnetic. |
 |
| Looking in just the xy plane, we see that the
electron in the dxy nonbonding orbital is
repelled by chlorine's lone pair in its p-orbital,
weaking Cl's bond to the metal. |
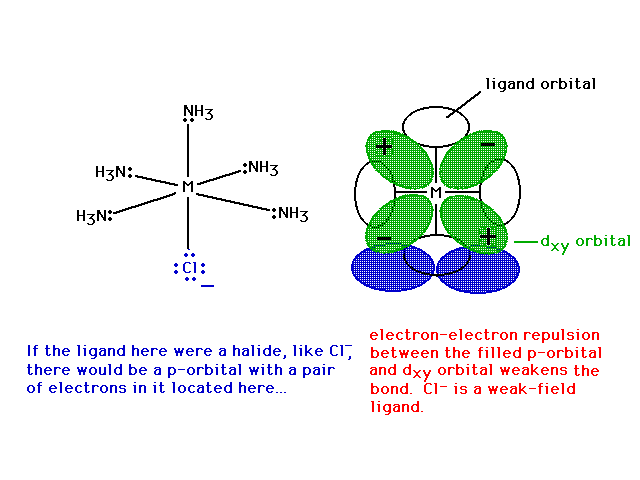 |
| Can we now explain why CO (and CN-) are strong field
ligands? |
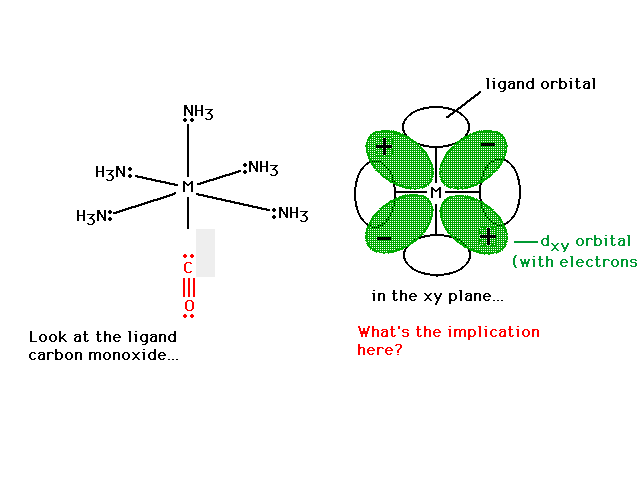 |
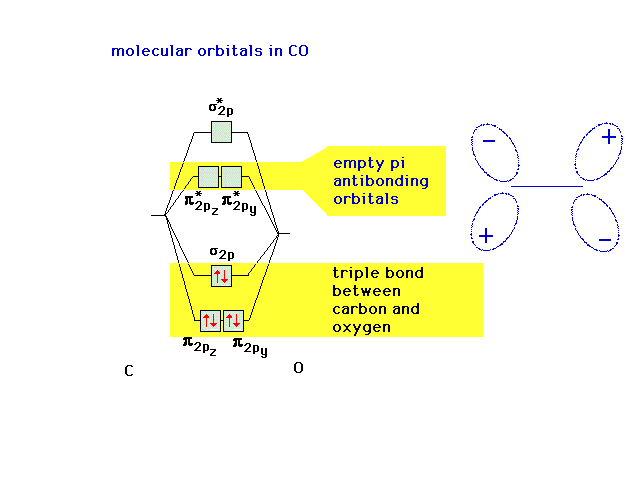
| Let's remind ourselves of the underlying valence
electron structure associated with CO, including the empty
anti-bonding molecular orbitals. |
 |
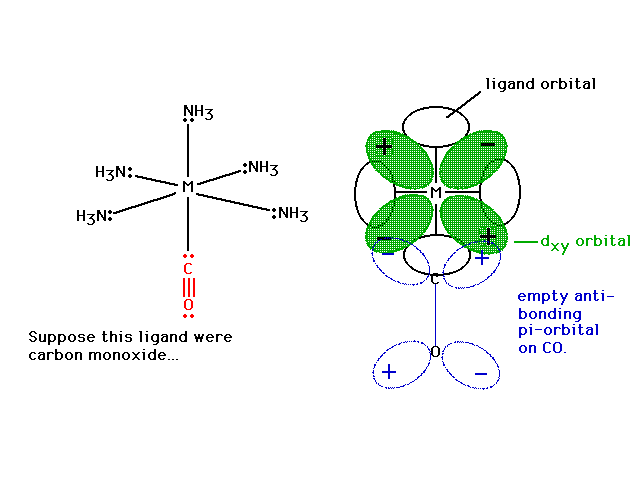
| With CO sigma bonded to the metal just as NH3
and Cl- were, the CO has its empty
pi-anti-bonding orbital placed so that a delocalized
molecular orbital in conjunction with the metal's dxy
becomes possible. Note the constructive interference
possibility. |
 |
| Here, then is the delocalized orbital into
which the previously though-to-be-non-bonding electron is
placed, giving the CO bond to the metal some partial
pi-bond character...making it a stronger bond! |
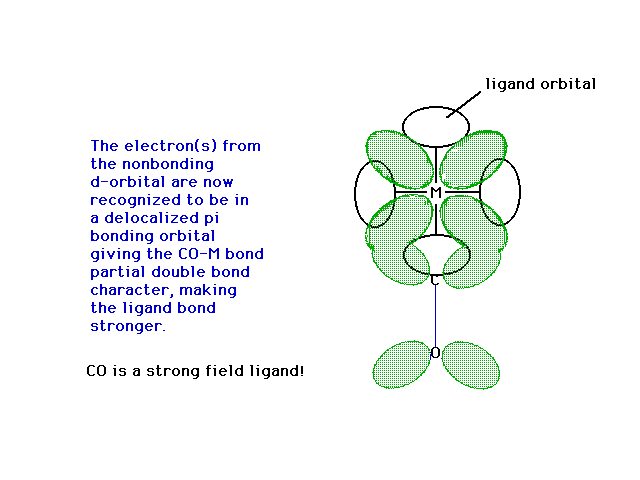 |
| ZnCl42- is the next example. |
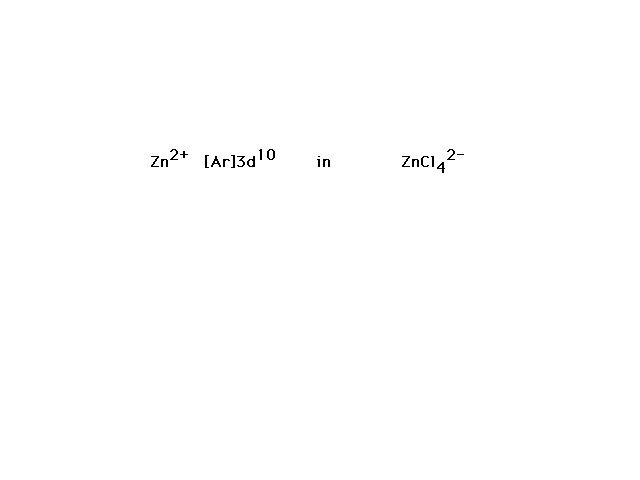 |
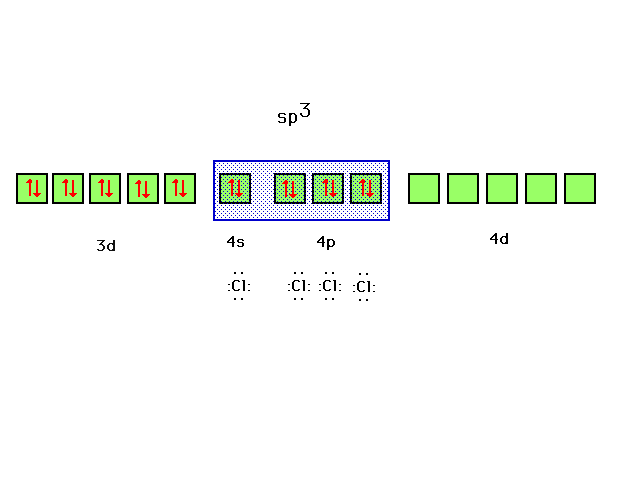
| Ten valence electrons fill the zinc 3d orbital. The
four ligands, each contributing a lone pair to bond
formation, utilize the hybrids formed from Zn's next four
orbitals, the 4s and the three 4p's corresponding to sp3
hybrids and the observed tetrahedral geometry for
complexes of this type. |
 |
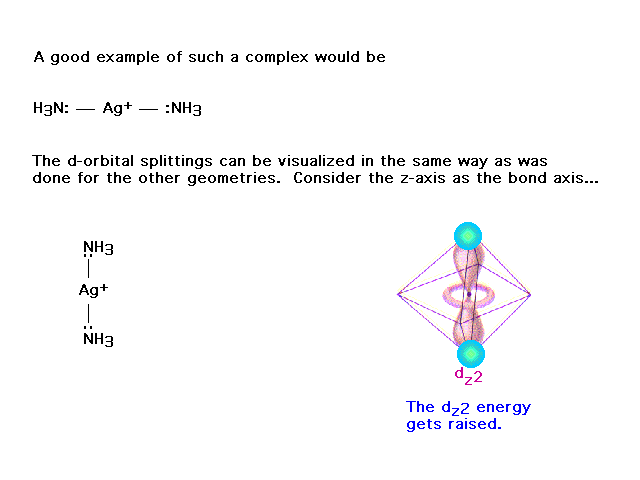
| (This was not included from an earlier lecture.)
Here's another attempt to show how crystal field
splitting works, but with coordination number 2, also
known as linear geometry. |
 |
| This orbital's electron, pointing directly at the
ligands (with their electrons) is raised in energy. |
 |
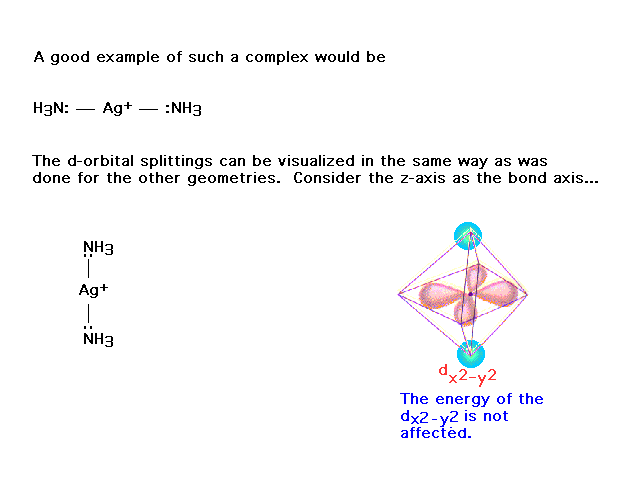
| On the other hand, this orbital does not point
towards the ligands and is unaffected by their presence.
Likewise for the dxy. |
 |