| Lecture
#32 |
| Text: Chapter 19, Sections 1, 3, 4. |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
Lecture Outline
|
Oxidation Numbers Transition
metals
Transition metal complexes
Stereoisomers
Geometrical isomers
Square planar geometries
Octahedral geometries
|
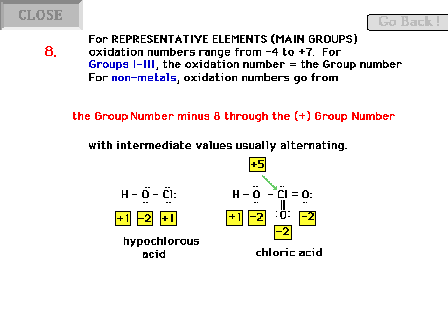
| Oxidation number rules continued. The rest of the
main group elements are covered by this rule. |
 |
| An oxidation number illustration of a salt in which
the structures of both ions is known and therefore
treated independently when assigning the oxidation
numbers. The computer-generated structures on the right
show the computer-generated electrostatic charges
actually at the different nuclei, thus illustrating that
oxidation numbers are an extreme intrepretation of the
effect of electronegativity differences on electron
deployment. |
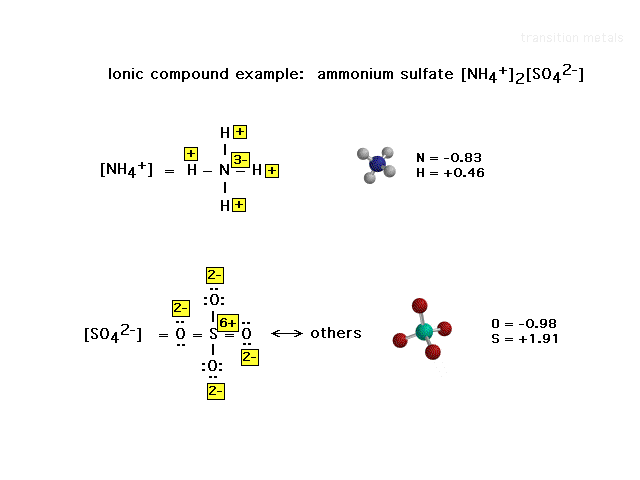 |
| Oxidation numbers need not be integers!! |
 |
Leaving the representative elements and looking at
the transition elements brings us back to d-electrons.
Transition metal chemistry |
 |
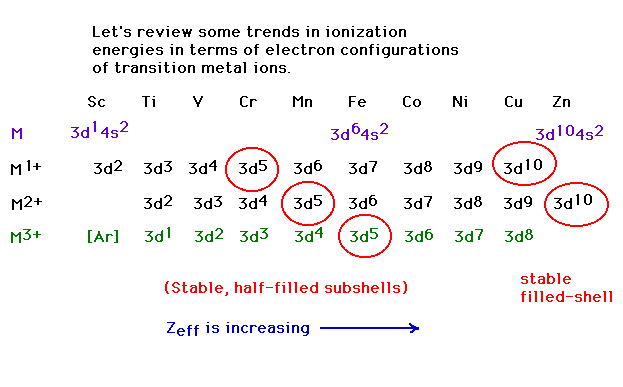
| Electron configurations of transition metal ions
(which do not follow the conventional order, as you
recall) |
 |
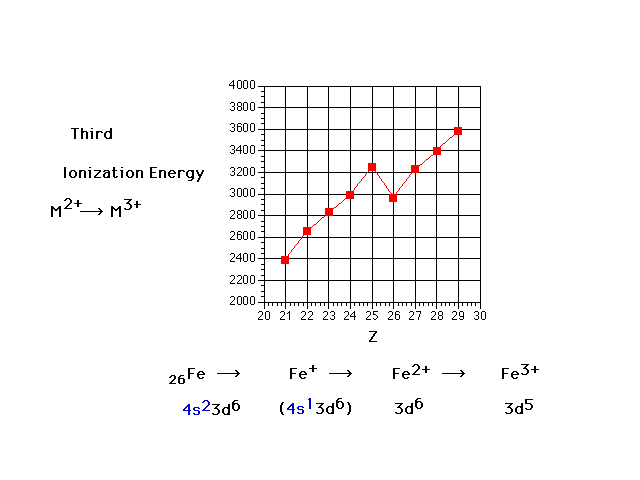
| Third ionization energies: the energy necessary to
remove a third electron from a transition metal. Why the
drop at Z=26 (iron)? |
 |
| Transition metal complex ions are transition metal
ions surrounded to bound ligands |
 |
| Donor atom illustration |
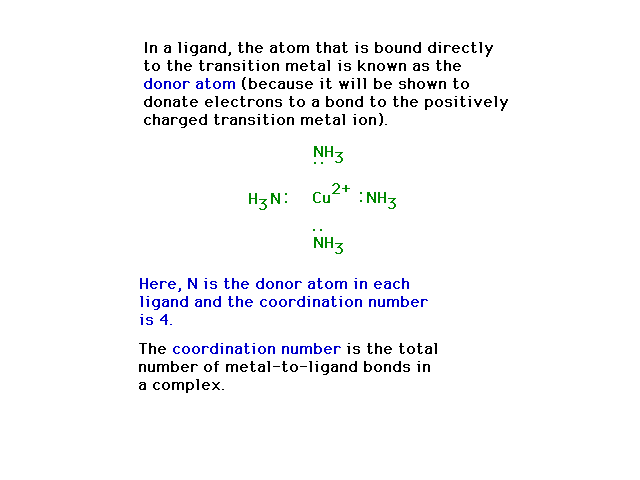 |
| The compound shown is a salt, and if dissolved in a
solvent (water) enables some revealing, simple
experiments to be done that help recognize something
about the molecule's structure. |
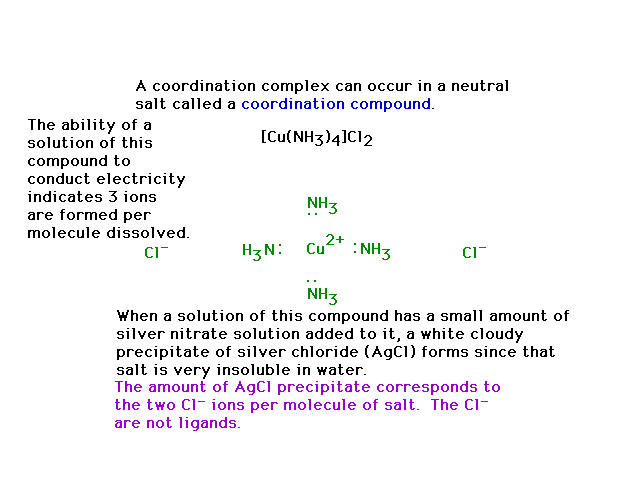 |
| A bidentate ligand connects to the central species
through two donor atoms from the same ligand. |
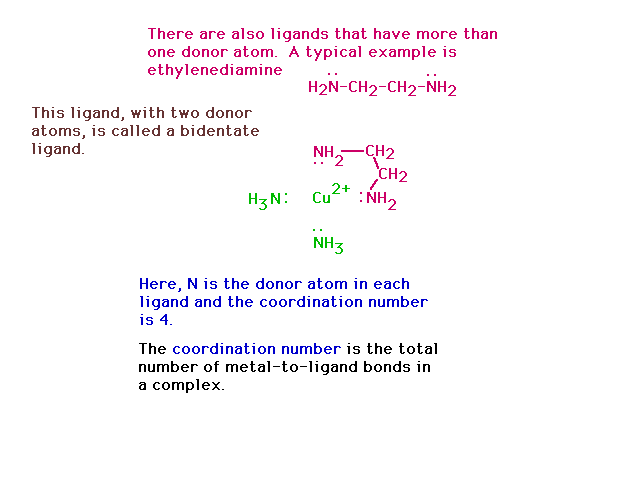 |
| Complex ions in compounds |
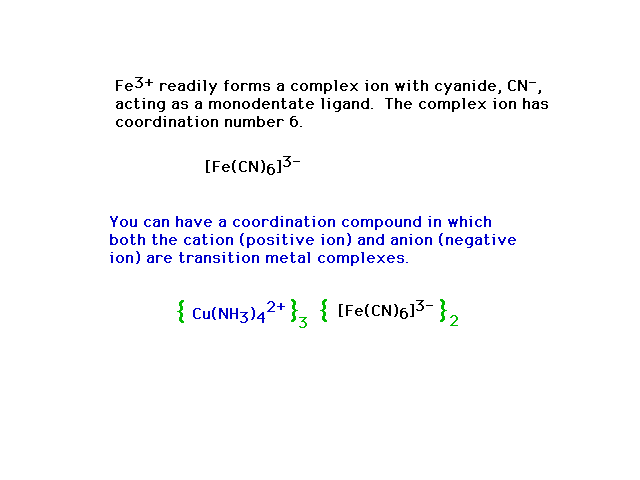 |
VSEPR was used to determine geometries when central atoms
were s and p block (main group) elements. VSEPR does not
work well when addressing the geometry of d block
(transition metal) elements. The geometries' we'll
consider, though, are limited as this slide indicates. |
 |
| After introducing the phenomenon of "waters of
hydration" and, separately, recalling structural and
geometrical isomerism, the following structural isomers
were listed, all with the formula Cr(H2O)6Cl3. |
 |
| Geometrical isomerism in octahedral complexes. The
bonds are indicated by green lines. Geometrical isomer
"trans" dichloro complex is on the left and a
"cis" dichloro complex is on the right. |
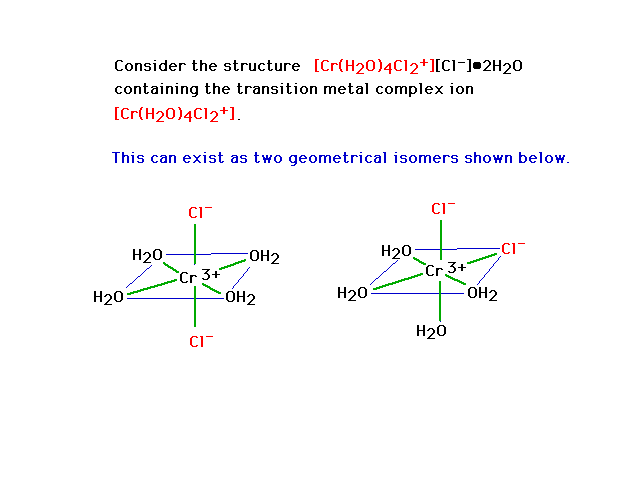 |
| More observations about geometrical isomerism in
octahedral complexes. |
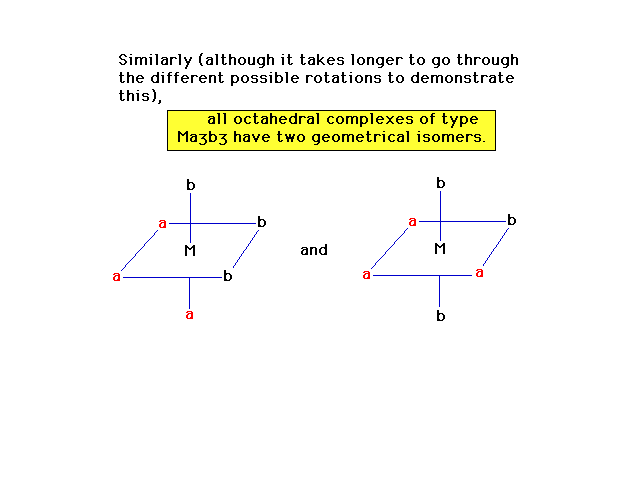 |
| More observations about geometrical isomerism in
octahedral complexes. |
 |
