| Lecture
#31 |
| This will move us to Chapter 19 |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Oxidation numbers Transition
Metal Chemistry
Tramsition metal ion electron configurations
Coordination complexes
Isomers
- Structural Isomers
- Stereoisomers
Geometrical isomers
|
Leaving the Representative (Main Group) elements and
looking at the transition elements brings us back to d-electrons.
Transition metal chemistry |
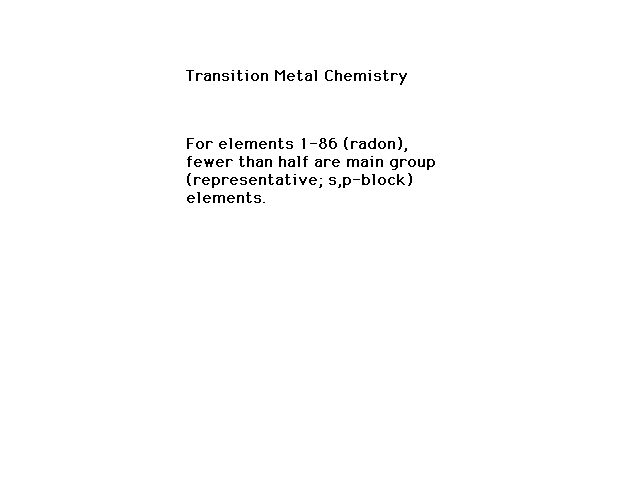 |
| Electron configurations of transition metal ions
(which do not follow the conventional order, as you
recall) |
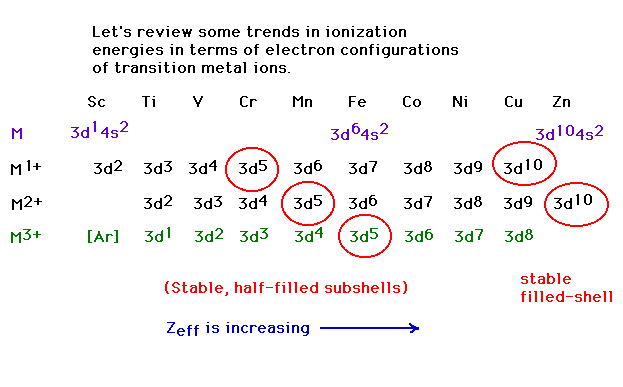 |
| Third ionization energies: the energy necessary to
remove a third electron from a transition metal. Why the
drop at Z=26 (iron)? |
 |
| Transition metal complex ions are transition metal
ions surrounded to bound ligands |
 |
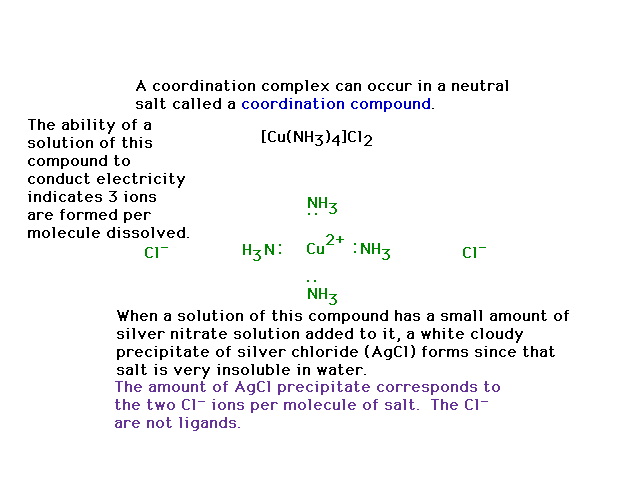
| The compound shown is a salt, and if dissolved in a
solvent (water) enables some revealing, simple
experiments to be done that help recognize something
about the molecule's structure. The experiments are
usually one of two types: electrical conductivity and
precipitation. |
 |
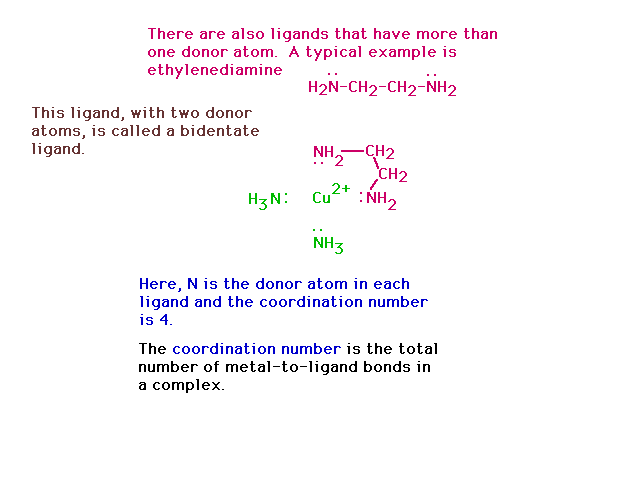
| A bidentate ligand connects to the central species
through two donor atoms from the same ligand. |
 |
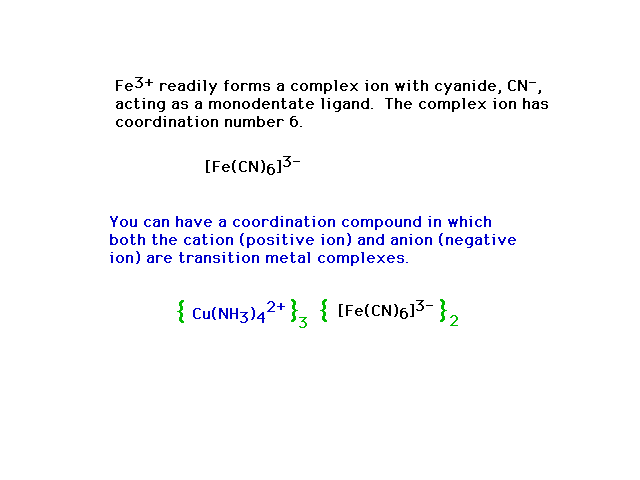
| Complex ions in compounds |
 |
VSEPR was used to determine geometries when central atoms
were s and p block (main group) elements. VSEPR does not
work well when addressing the geometry of d block
(transition metal) elements. The geometries' we'll
consider, though, are limited as this slide indicates. |
 |
| After introducing the phenomenon of "waters of
hydration" and, separately, recalling structural and
geometrical isomerism, the following structural isomers
were listed, all with the formula Cr(H2O)6Cl3. |
 |
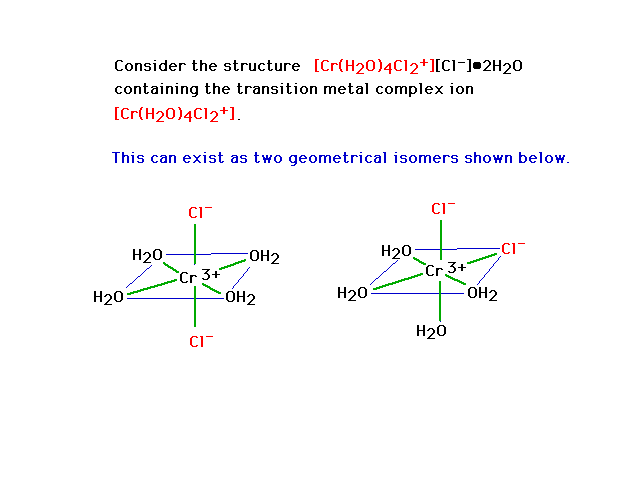
| Geometrical isomerism in octahedral complexes. The
bonds are indicated by green lines. Geometrical isomer
"trans" dichloro complex is on the left and a
"cis" dichloro complex is on the right. |
 |
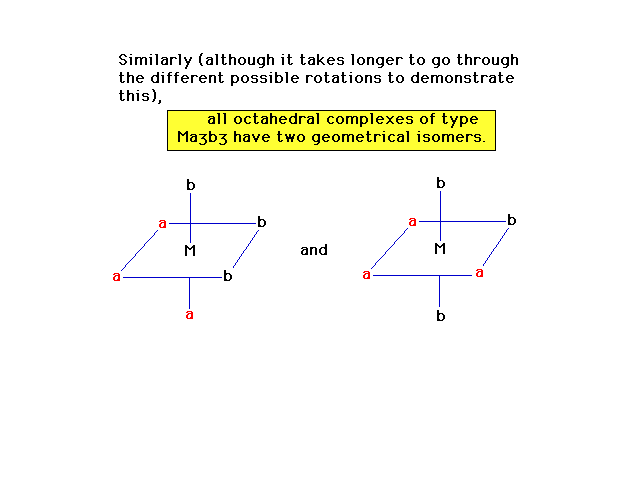
| More observations about geometrical isomerism in
octahedral complexes. |
 |
| More observations about geometrical isomerism in
octahedral complexes. |
 |
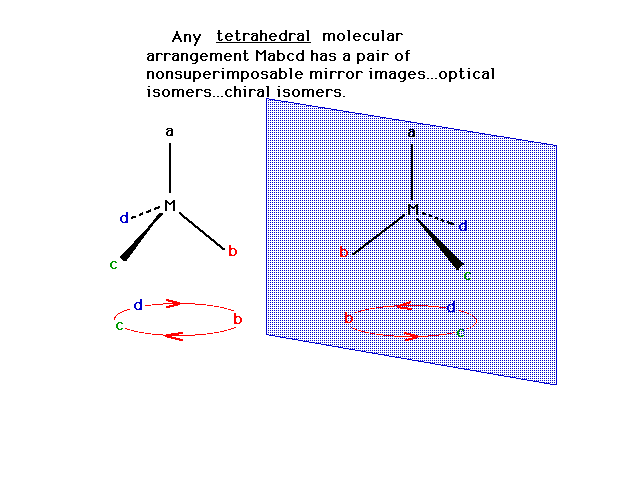
| A class of isomers called "stereoisomers"
consists of geometrical isomers, which we already have
seen examples of, and "optical isomers", also
called "enantiomers". Optical isomers are said
to be "chiral" and have a distinction between
them that is similar to the distinction between the left
hand and the right hand. |
 |
| A tetrahedral arrangement symbolized as Mabcd (four
different ligands) consists of two possible optical
isomers. 
|
 |