| Lecture
#17 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Start of Review for Exam II |
| List of topics for EXAM II. |
 |
| Additionally, a question from Exam II from the Spring
of 2000 will be gone over to give more examples
clarifying the distinction between structural and
geometrical isomerism |
 |
| |
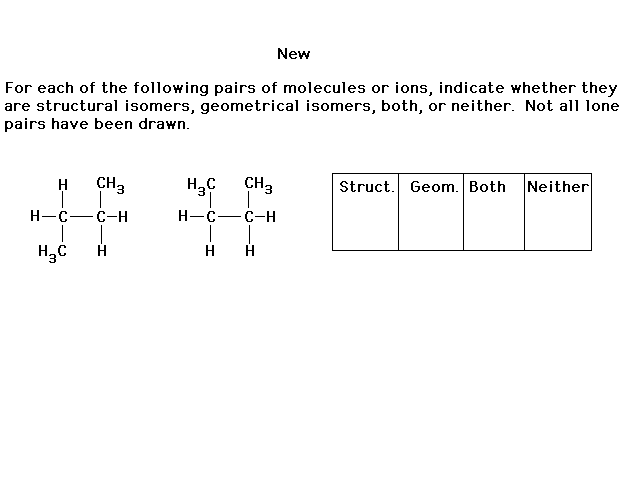 |
| According to our growing understanding of structural
isomerism, these two choices represent two different
frameworks. For example, in the positive ion, the top Pt
is bonded to four ammonias. The lower Pt is bonded to
three ammonias and one chloride. These are structural
isomers. |
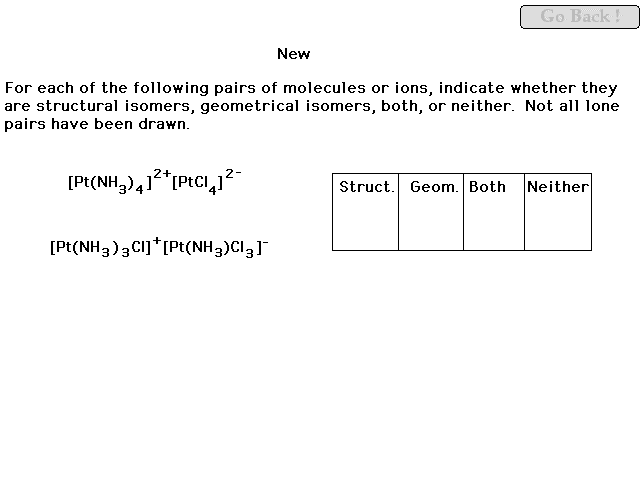 |
| This comparison will be gone over thoroughly in the
review. |
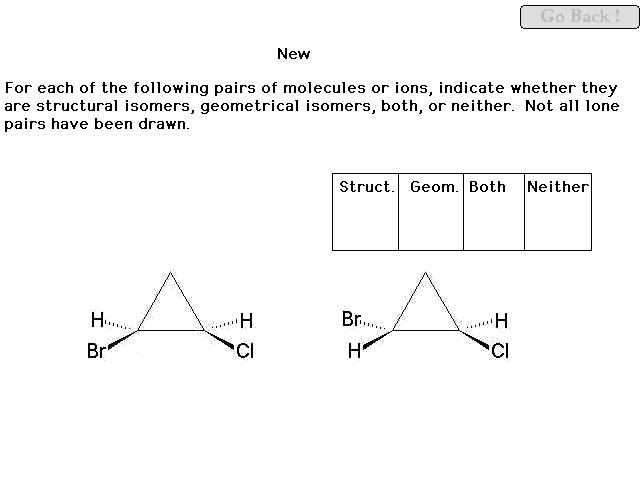 |
| Molecular basis of acid strength |
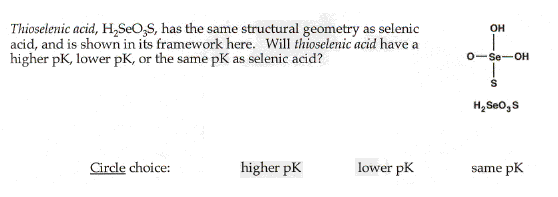 |




