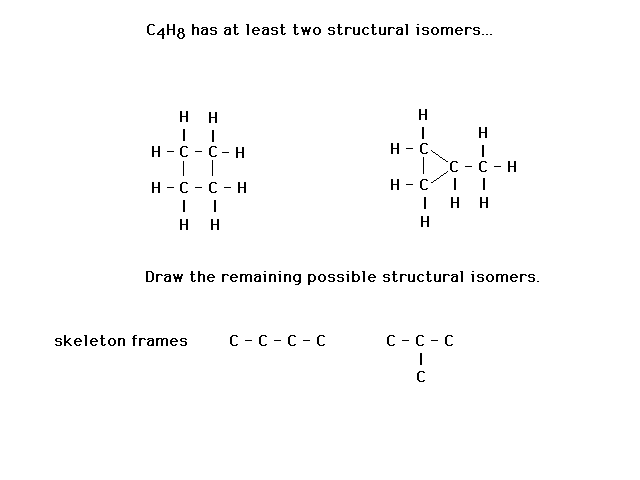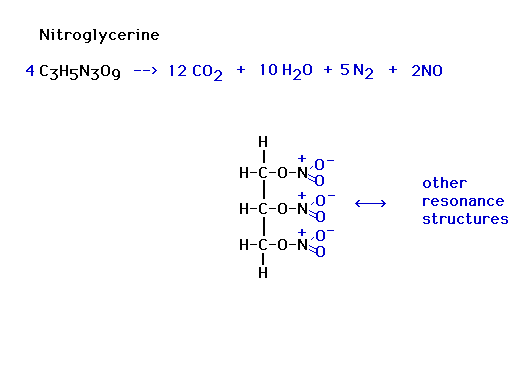| Lecture
#19 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Review for Exam II |
| List of topics for EXAM II. |
 |
| An example of one of five structural isotopes of C4H8
followed a "recipe" (not strongly encouraged)
for arriving at its Lewis structure. |
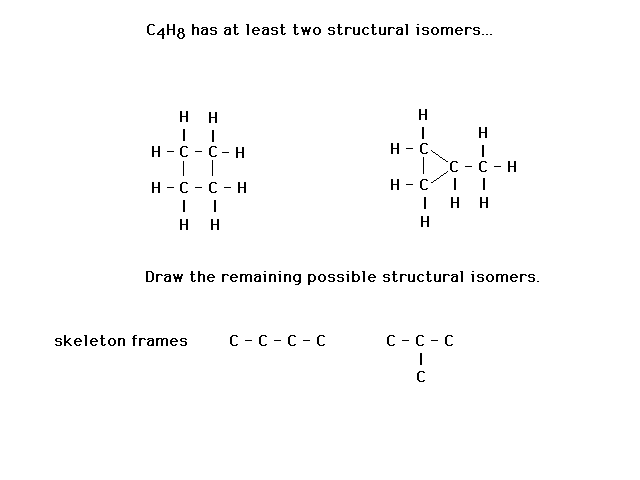 |
| Examples: (Reaction Heat...think of it as balancing a
financial account, reactants require energy to break up,
products supply energy to do this, what energy makes this
all balance?) Energy released or energy input to the
reactants? |
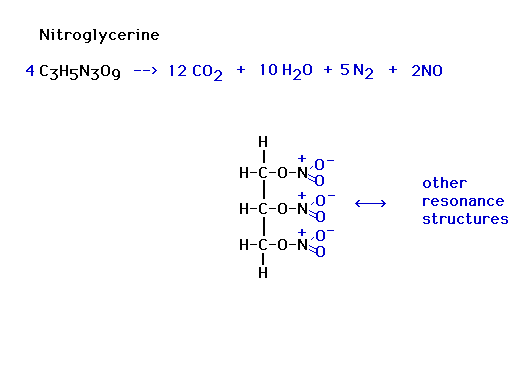 |
| We will use VSEPR to determine bond angles in a
collection of different illustrations some of which are
shown here. |
 |