| Lecture
#13 |
| Text: Section 13.3 |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Lewis structures
Reaction Heats
Dipole moments and bond polarity
Line Structures
|
| An application: We'll diverge
slightly and discuss bond energies and one way in which
they can be applied, the energy released (reaction
heat) in the combustion of octane. Here we get the
balanced equation for the complete combustion reaction.
(Reaction heats are not covered in the
text.) |
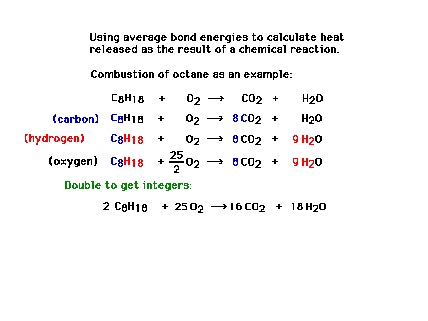 |
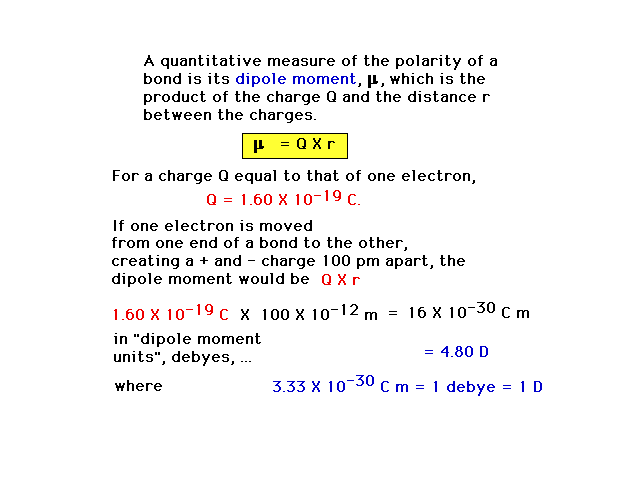
| A purely covalent bond, with perfectly shared valence
electrons, has no "polarity" or separation of
charges. The degree to which a bond has polarity can be
expressed by a dipole moment, defined
here (but not in the text.). |
 |
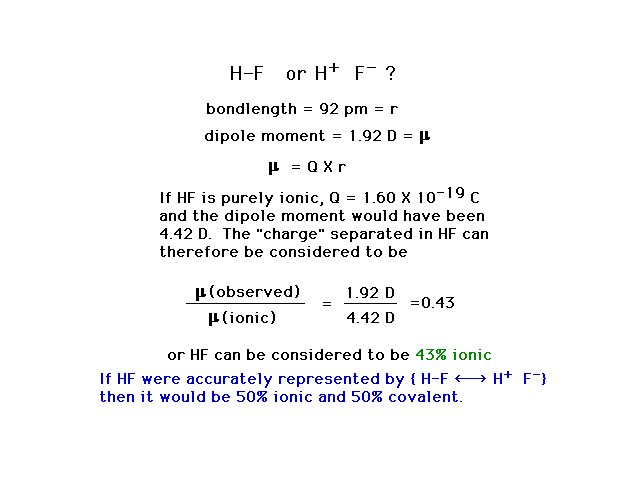
| Calculating the "percent ionic character"
or "partial ionic character" for the diatomic
molecule HF using the measured dipole moment and the
measured bond length. |
 |
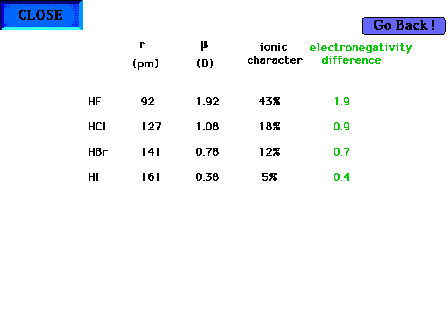
| The percent ionic character for the hydrogen halides.
You should be able to calculate the %'s from the various
bondlengths and dipole moments. Note that the percent
ionic character decreases as the halogen becomes less
electronegative, leading to an almost purely covalent
bond (covalent = 0% ionic) for HI.You might want to try
one of these. Percent ionic character is not in our text. |
 |
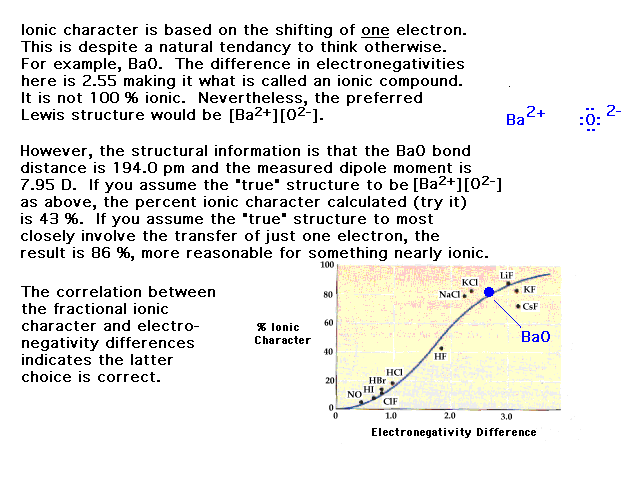
| (Probably not discussed in lecture and not critical,
but some may ask about it...) The question always arises
about what to do if the compound in question might be
considered as X2+Y2-. Is the
percent ionic character based on two units of charge? The
chatter here suggests that as soon as you get +1
separated from -1, we're talking about an ionic
situation. It won't come up in the reading, in quizzes,
or exams, but it is worth dealing with as an aside here. |
 |
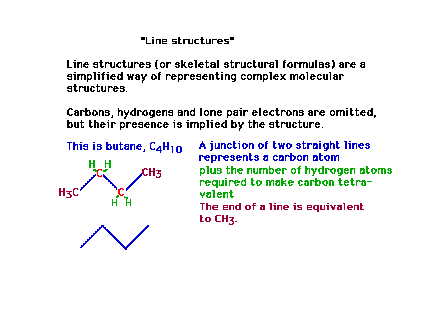
| Line structures are a useful and very common
way of presenting Lewis structures. They are essentially
geometric abbreviations. |
 |
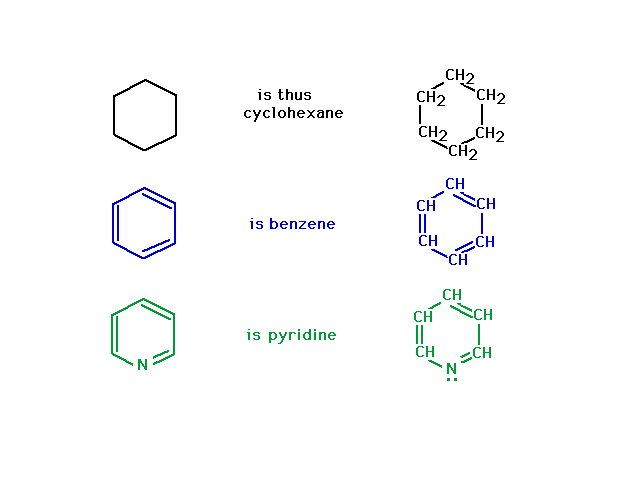
| Here are two illustrations of line structures
that look similar, but aren't quite the same. |
 |
