| Lecture
#10 |
| Finishing Chapter 9 now |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Electron Configurations (continued)
Ionization energies
Second and third ionization energies
Electron Affinity
Valence electrons and electron dot structures in
atoms
|
| The energy needed to remove the easiest-to-remove
electron from a neutral atom is called the first
ionization energy. |
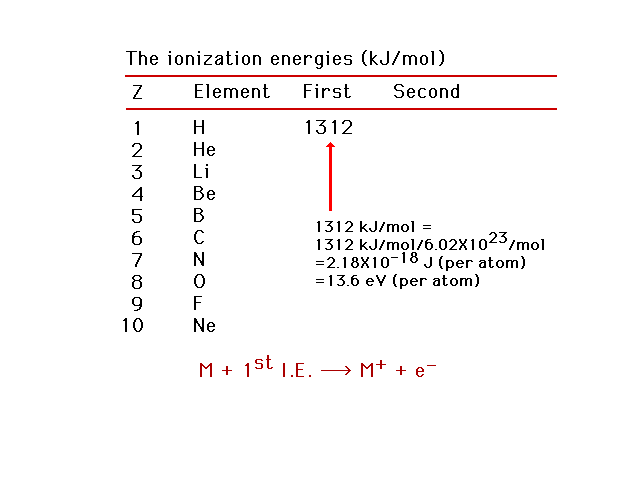 |
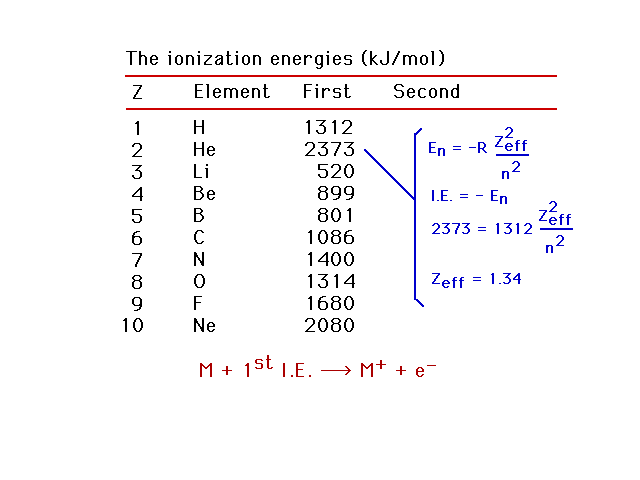
| First ionization energies for light elements. Using
this information, we can estimate the effective nuclear
charge, Zeff, for the electron being removed.
Helium as an example. |
 |
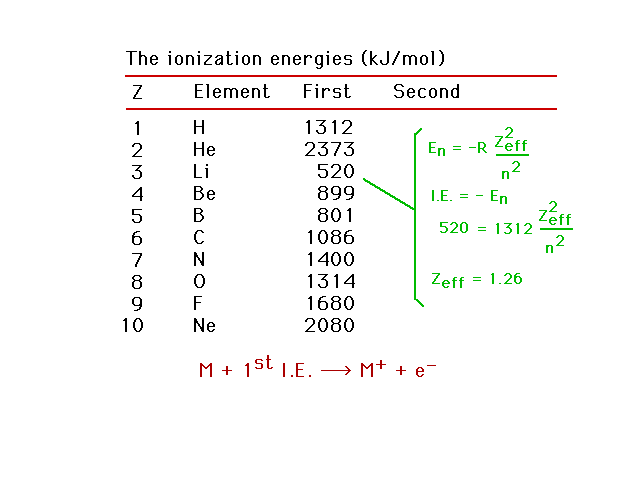
| Zeff for lithium |
 |
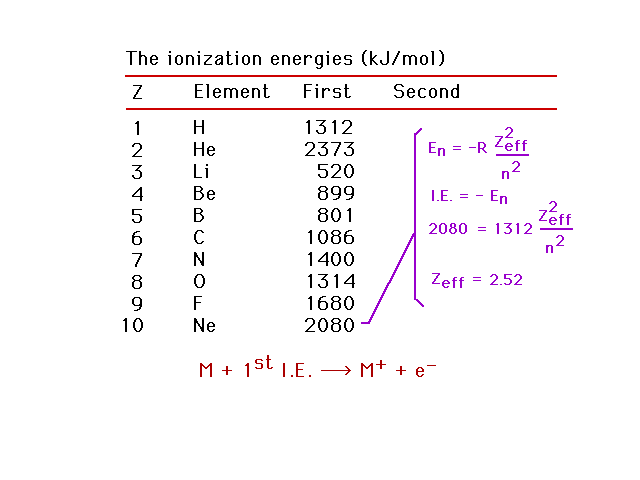
| Zeff for neon |
 |
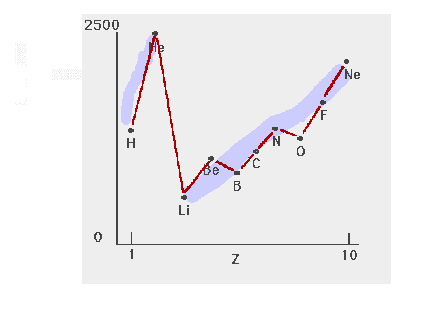
| The detailed trend in ionization energies for the
light elements |
 |
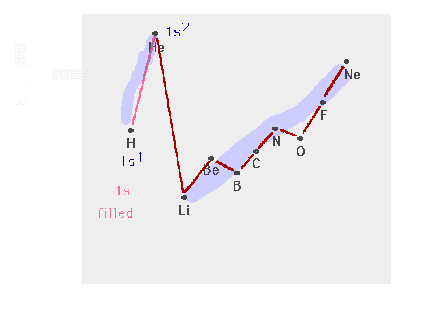
| The n=1 shell filling |
 |
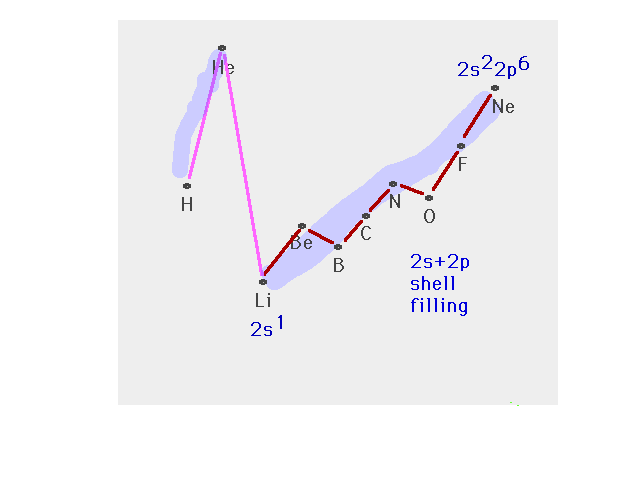
| The n=2 shell filling after which the n=3 shell
starts |
 |
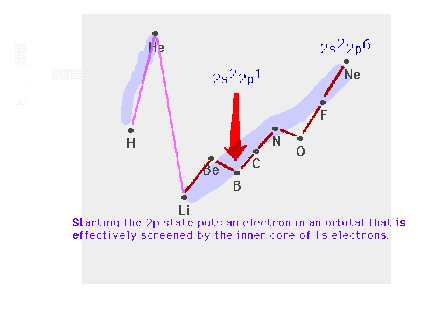
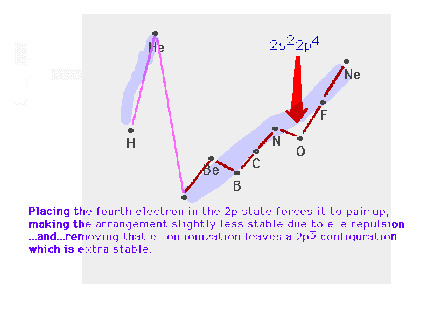
| Starting the p-subshell causes a break in the smooth
trend across the row. |
 |
| Starting to pair up electrons after half the
p-subshell is filled causes a second break, which we
referred to as the mid-shell dip, in the smooth trend
across the row. |
 |
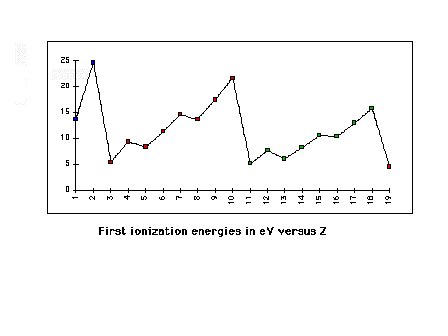
| First ionization energies across rows 1 through 3 of
the Periodic Table |
 |
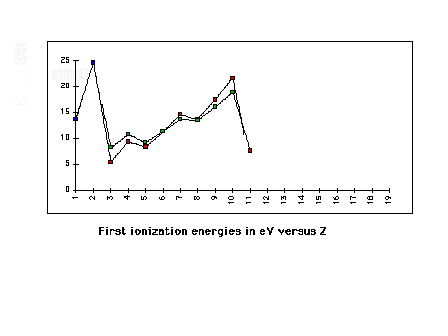
| Overlapping the 2nd and 3rd row element ionization
energies to demonstrate the repeating pattern (determined
by valence electron configuration) |
 |
| Second ionization energies |
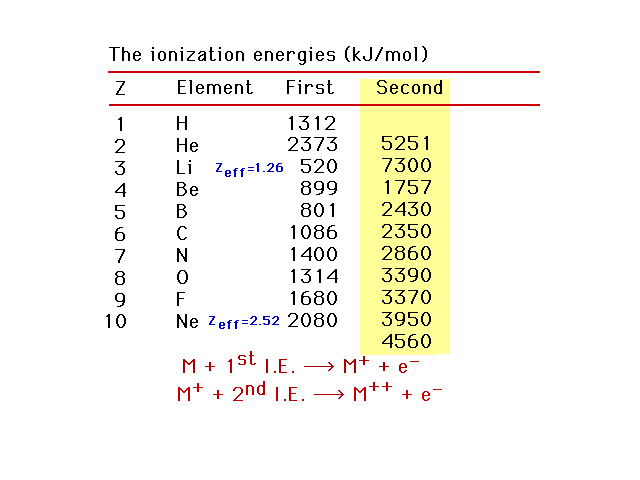 |
| First, second, and third ionization energies for the
light elements |
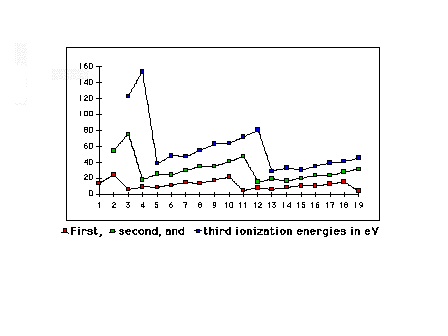 |
| First, second, and third ionization energies shifted
to show, again, that valence electron configuration is
the determining driver |
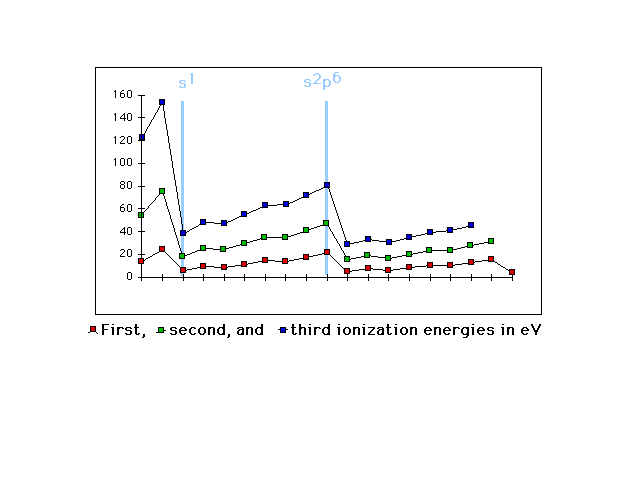 |
| Electron affinity is the energy involved in
adding an electron to a neutral atom to form a negative
ion. It is numerically equal to minus the ionization
energy for that negative ion. As such, we should expect
that the electron affinities also depend on electron
configuration. |
 |
| A pictorial schematic of valence electrons for the
Main Group (s- and p-block) elements is shown here where
each "dot" represents an accounting of a
valence electron. The scheme was invented by G. N. Lewis
and these are referred to as Lewis electron dot pictures
of elements. |
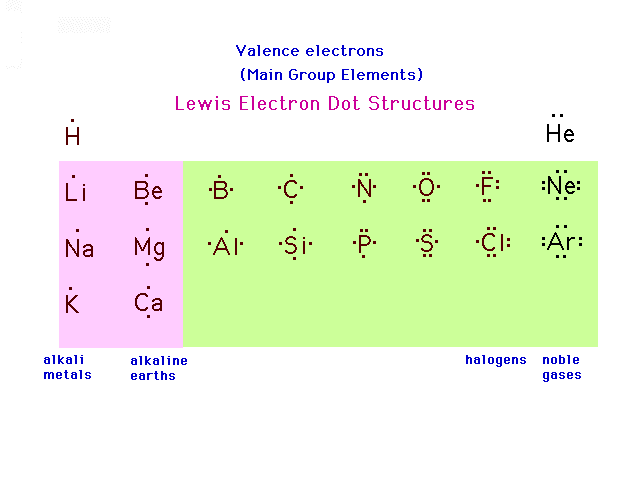 |
| Electronegativity, a property of atoms that will
become important in our discussion of molecules. |
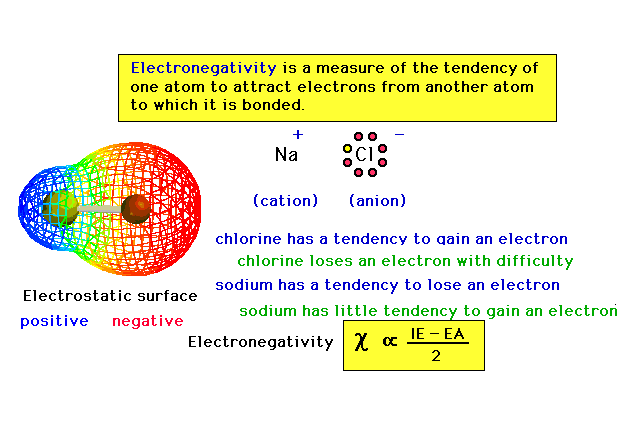 |
