| Lecture
#10 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Electronegativity Molecular
Structure
Lewis Structures
Ionic compounds
|
| Periodic trends of electronegativity of the elements
through the first five rows. |
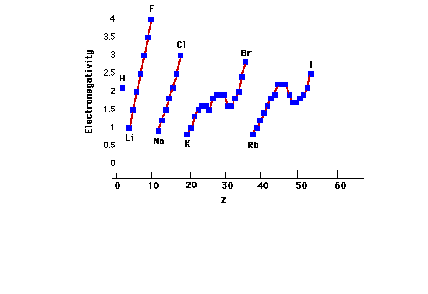 |
| A pictorial schematic of valence electrons for the
Main Group (s- and p-block) elements is shown here where
each "dot" represents an accounting of a
valence electron. The scheme was invented by G. N. Lewis
and these are referred to as Lewis electron dot pictures
of elements. |
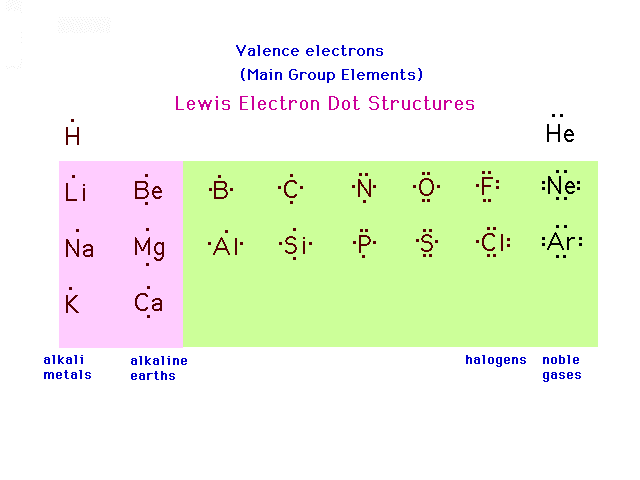 |
| Electronegativity, a property of atoms that will
become important in our discussion of molecules. |
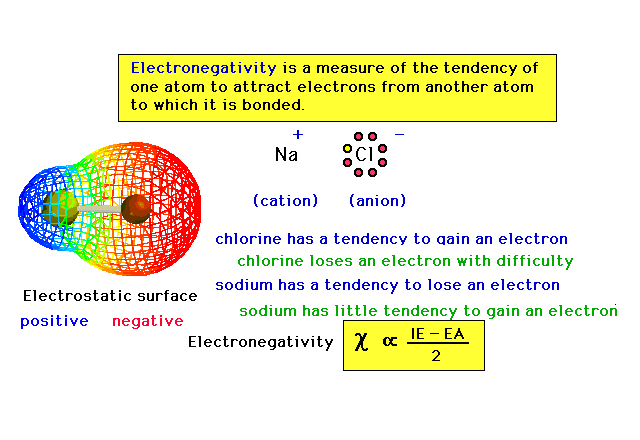 |
| As above, but just for the s- and p-block (main
group) elements. |
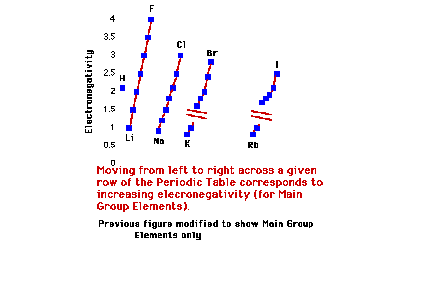 |
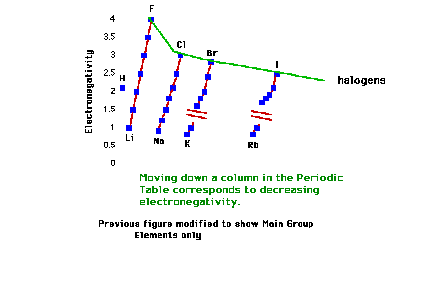
| Electronegativity trend of the halogens |
 |
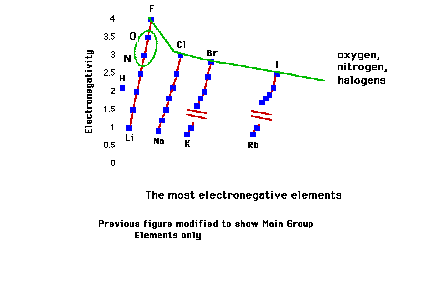
| The most electronegative elements. |
 |
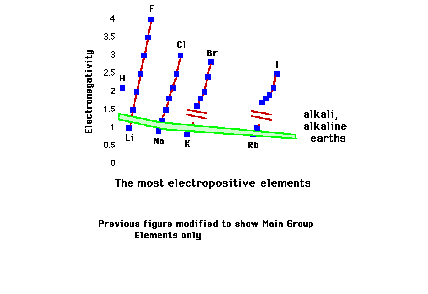
| The least electronegative elements. |
 |
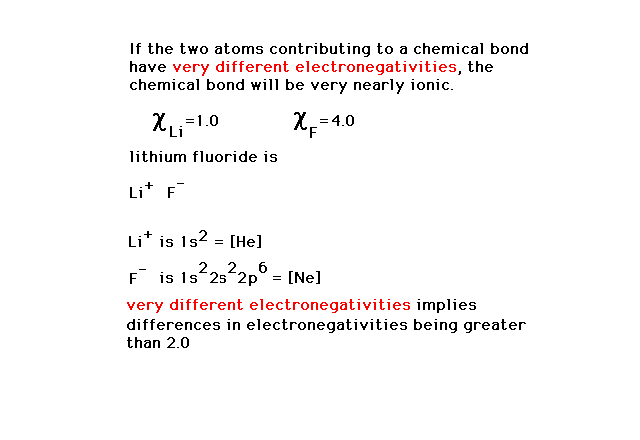
| The compounds formed between very electronegative
atoms and very electropositive atoms will invariable be
ionic in nature. Lithium fluoride is a prime example of
this. |
 |
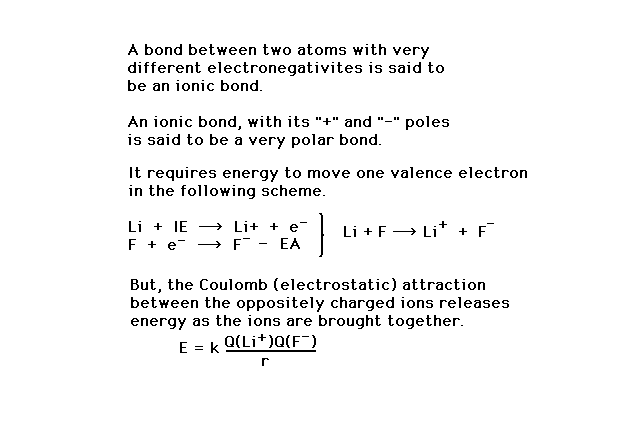
| The strength of an ionic bond can be understood as
due to a hypothetical assembly of the atoms involved.
Ionizing Li and putting the released electron onto a
separated fluorine atom involves IE(Li) and EA(F). Since
IEs are generally much larger than EAs, this dominates
what will be a cost in energy. Energy is the released as
the potential energy of electrostatic attraction between
the Li+ and F- is taken advantage
of by bringing the ions close together (to their eventual
bond separation distance). |
 |
| Since even greater energy would be returned if Li2+
and F2- were brought together, you can
convince yourself that the cost in making these two ions
is exceptionally high. Look at electron configurateions. |
 |
| In combining atoms to form molecules, Lewis' Octet
Rule accounts for how valence electrons are distributed.
Note this is not a theory to explain bonding, but
merely a book-keeping scheme for tracking valence
electrons consistent with what is observed in molecular
structure. |
 |
| An example of sharing valence electrons to
accommodate the Octet Rule. |
 |
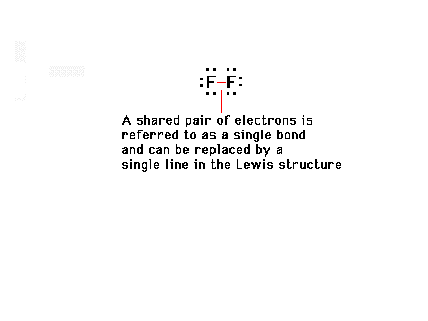
| In symbolizing valence electrons, a single line can
be used to represent a shared pair of electrons; that is,
to represent one bonding pair of electrons. |
 |
| More than one pair of electrons can be shared to be
consistent with the octet rule. |
 |
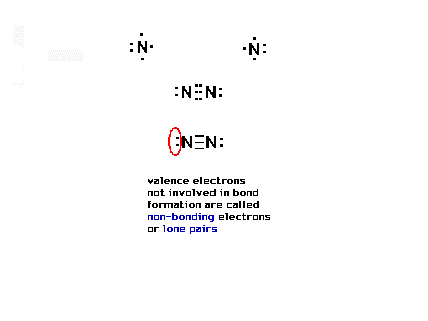
| Here, there are three shared pairs of electrons (six
electrons involved) and also two pairs of electrons not
involved in bonding at all, and called "lone
pairs". |
 |
| These are a small selection of bond energies showing
how the bond energy increases with increasing bond
multiplicity |
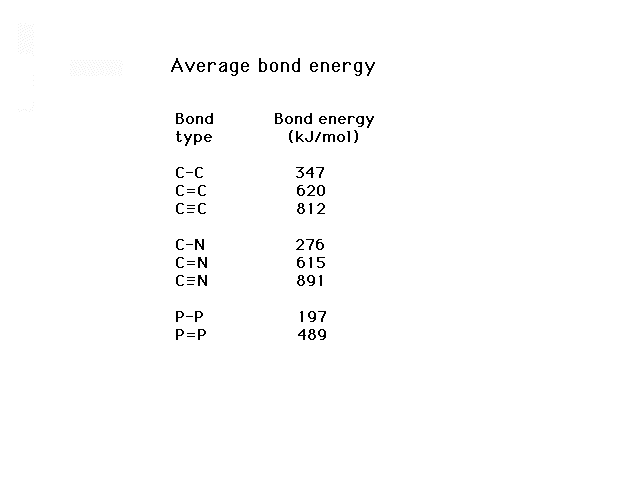 |
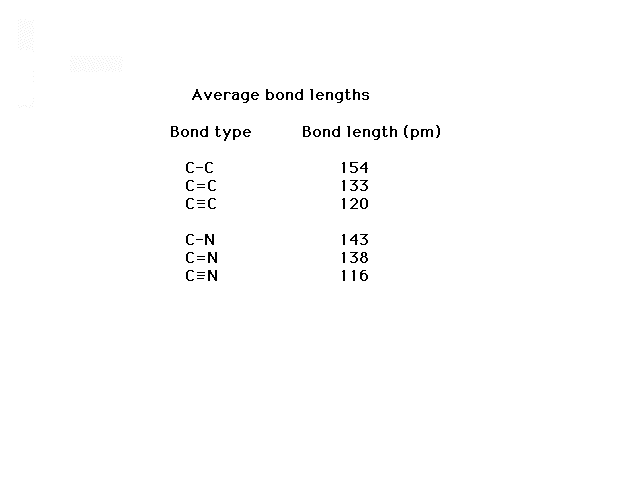
| These values illustrate how bond lengths decrease
with increasing bond order. |
 |
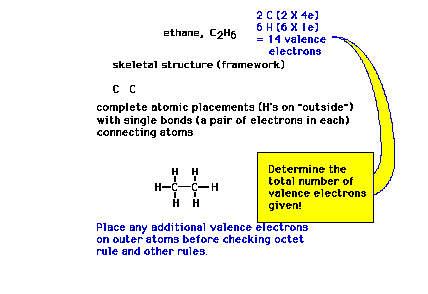
| More than two atoms: When given a molecular
formula for a polyatomic molecule, such as ethane, the
very first step in constructing the complete Lewis dot
structure is to arrive at a framework, a skeleton, of how
the nuclei are linked. |
 |
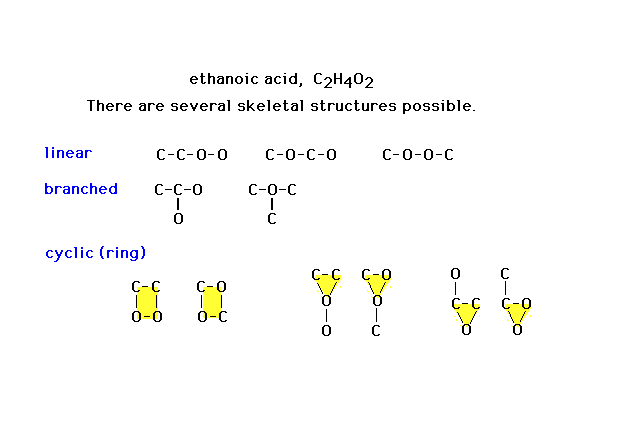
| Here are eleven different skeletal structures for a
molecule with the formula shown. Not all will prove to be
relevant. Some will not work at all as frameworks to be
completed. (Note added, one more linear possibility is
missing: that with oxygen at each end.) |
 |
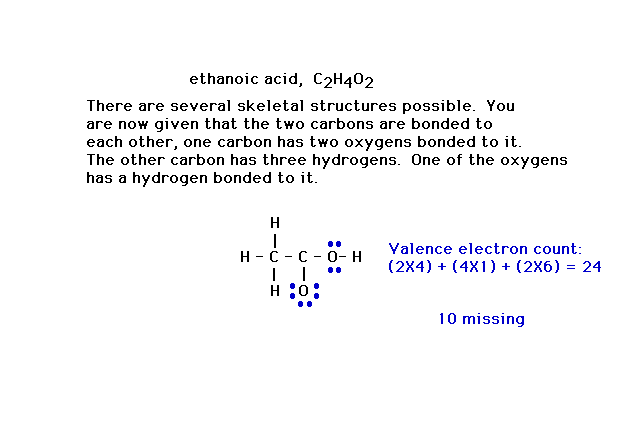
| Here's a slightly more complicated situation in which
the formula, C2H4O2, has
a number of different skeletal structures possible.
Additional information allows you to draw the framework
shown in which the linkages have used 14 valence
electrons. |
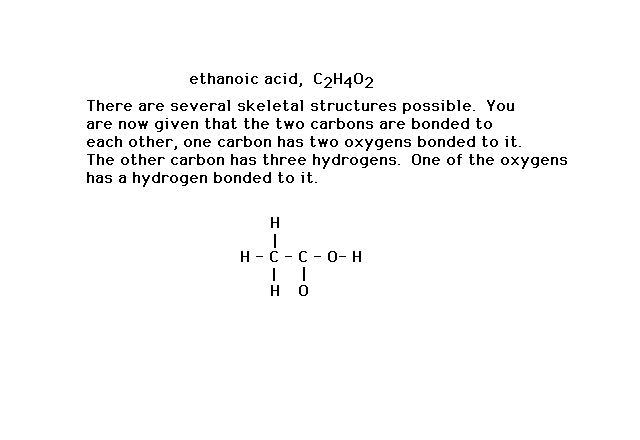 |
| Ten more valence electrons must be placed in the
structure. They are placed in the atoms whose octets are
not yet filled. Ten such electrons are placed here in
blue. Other arrangements are possible, but not shown. The
carbon in the C-O has only six electrons and violates the
octet rule. A pair of electrons from neighboring oxygen
can be moved over and shared with that carbon. |
 |
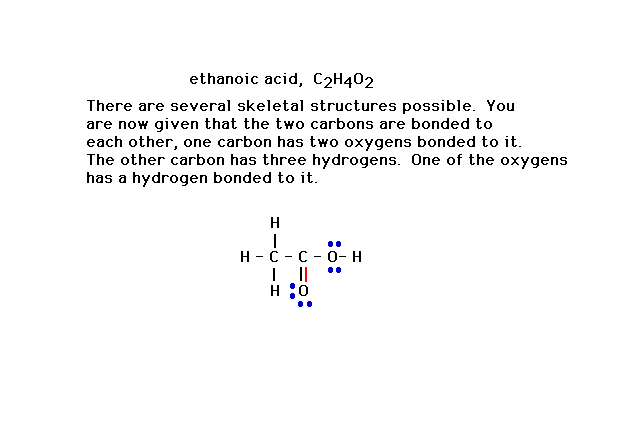
| This is the correct Lewis structure for the compound
identified. (Alternatively, a pair of electrons from the
other oxygen would have satisfied the octet rule as well,
but another consideration -- formal charge -- would
reject that possibility as less favorable. We discuss
formal charge later in the lecture. |
 |




