| Lecture
#11 |
| Chapter 13 in text now. We will
follow a different sequence than the text. Text:
Sections 13.2 and 13.10
|
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Molecular Structure
Lewis Structures
Valence electrons (Main groups)
Octet Rule
|
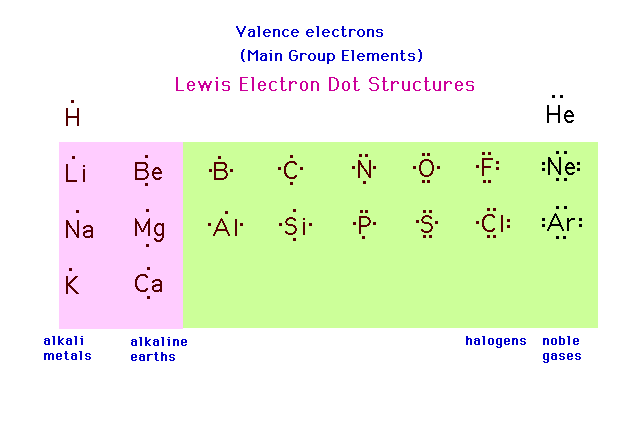
| The valence electrons can be represented by an
"electron dot" around the symbol of each
element. These are conventionally indicated either as
separate single dots or as pairs, depending on the total
number of valence electrons as shown in the figure. |
 |
| In combining atoms to form molecules, Lewis' Octet
Rule accounts for how valence electrons are distributed.
Note this is not a theory to explain bonding, but
merely a book-keeping scheme for tracking valence
electrons consistent with what is observed in molecular
structure. |
 |
| An example of sharing valence electrons to
accommodate the Octet Rule. |
 |
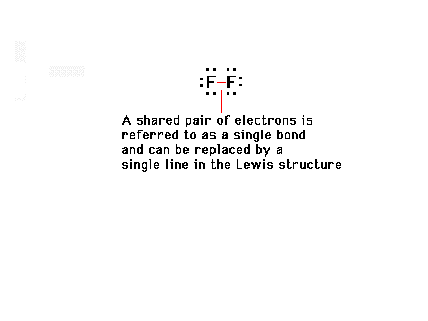
| In symbolizing valence electrons, a single line can
be used to represent a shared pair of electrons; that is,
to represent one bonding pair of electrons. |
 |
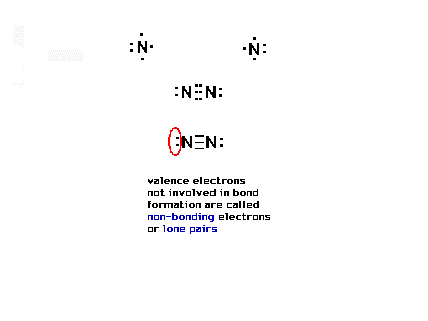
| More than one pair of electrons can be shared to be
consistent with the octet rule. |
 |
| Here, there are three shared pairs of electrons (six
electrons involved) and also two pairs of electrons not
involved in bonding at all, and called "lone
pairs". |
 |
| These are a small selection of bond energies showing
how the bond energy increases with increasing bond
multiplicity |
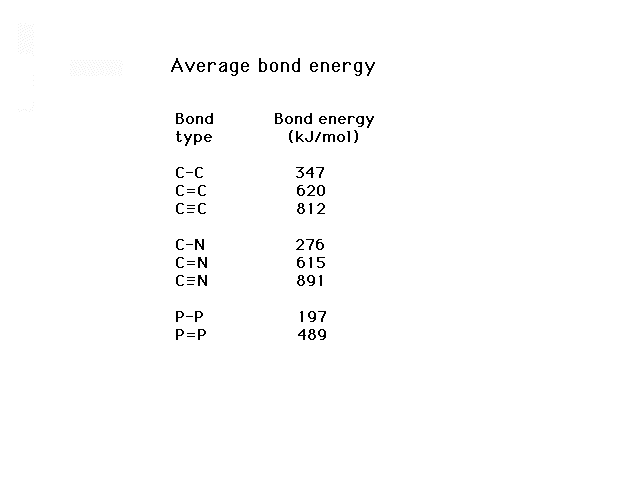 |
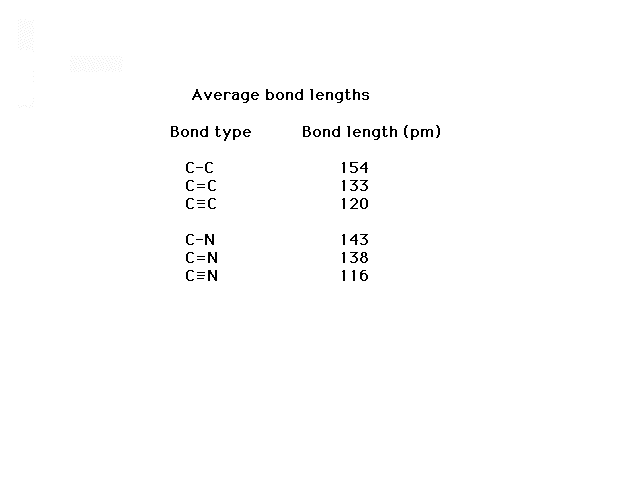
| These values illustrate how bond lengths decrease
with increasing bond order. |
 |
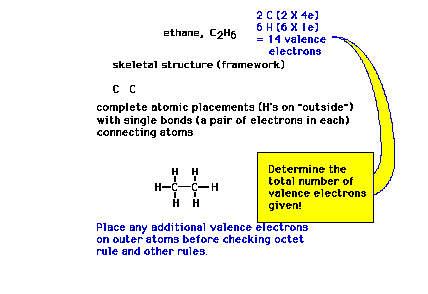
| More than two atoms: When given a molecular
formula for a polyatomic molecule, such as ethane, the
very first step in constructing the complete Lewis dot
structure is to arrive at a framework, a skeleton, of how
the nuclei are linked. |
 |
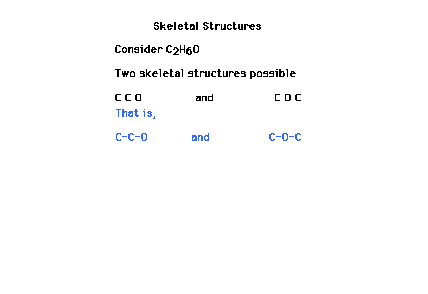
| One of the first steps in constructing a Lewis dot
structure is to decide on the "skeletal"
arrangement of key atoms. Hydrogen and fluorine are
(almost) always on the outside and thus not part
of the skeleton. |
 |
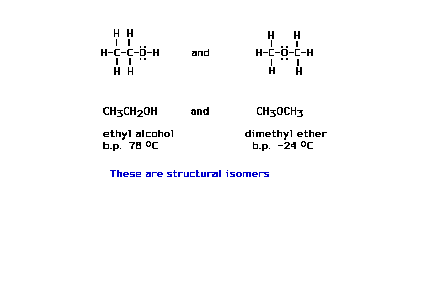
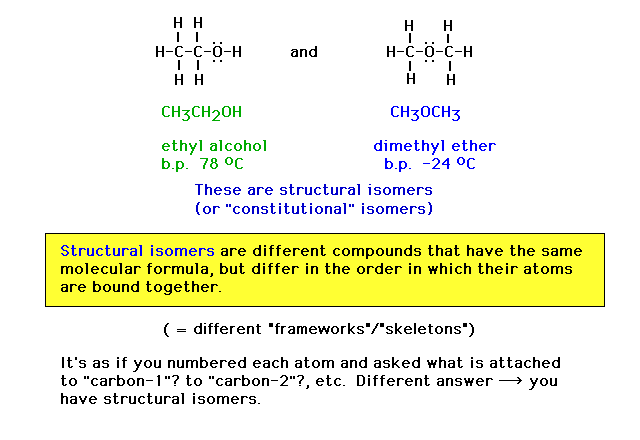
| Here are two different chemical species, each with
exactly the same molecular formula. They are the fairly
common substances ethanol and dimethyl ether. (b.p.
abbreviates boiling point) |
 |
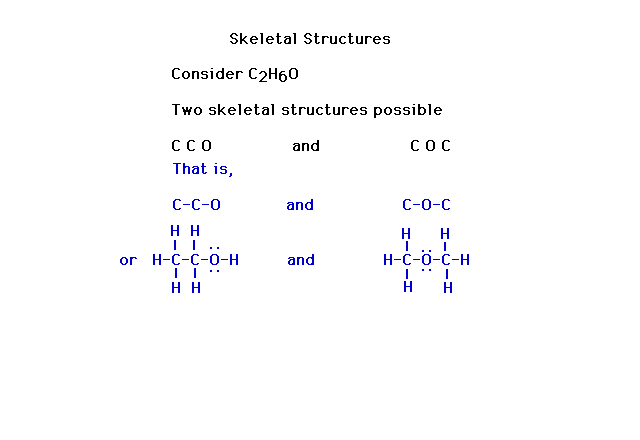
| Without skeletal information, there can be more than
one result in figuring out a Lewis electron dot
structure. |
 |
| Here we introduce the term "structural
isomers" using the above illustration. |
 |
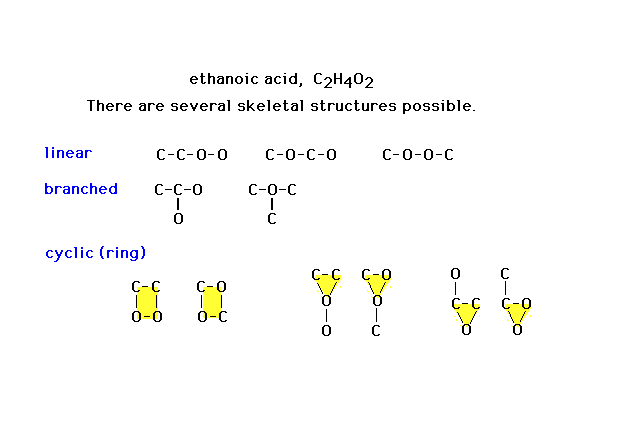
| Here are eleven different skeletal structures for a
molecule with the formula shown. Not all will prove to be
relevant. Some will not work at all as frameworks to be
completed. (Note added, one more linear possibility is
missing: that with oxygen at each end.) |
 |
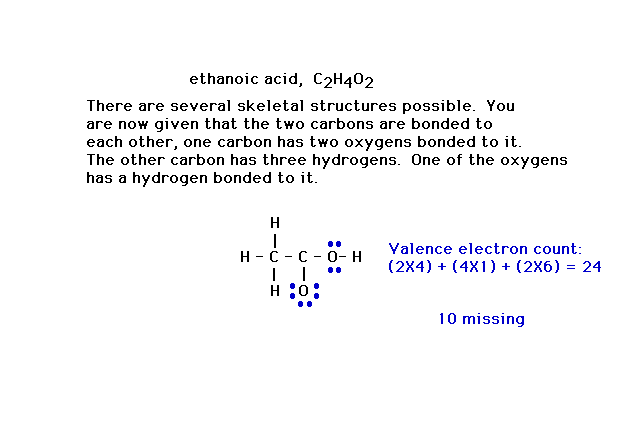
| Here's a slightly more complicated situation in which
the formula, C2H4O2, has
a number of different skeletal structures possible.
Additional information allows you to draw the framework
shown in which the linkages have used 14 valence
electrons. |
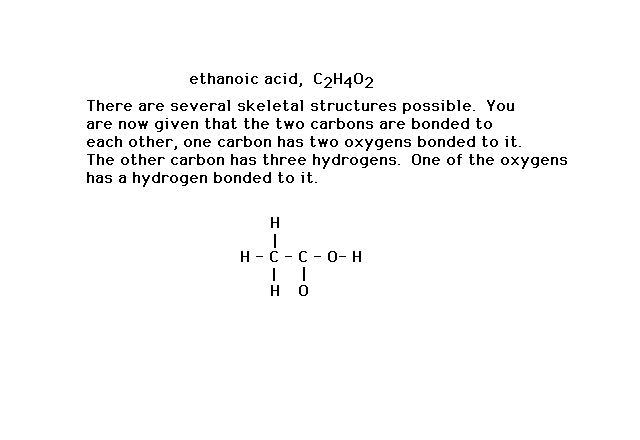 |
| Ten more valence electrons must be placed in the
structure. They are placed in the atoms whose octets are
not yet filled. Ten such electrons are placed here in
blue. Other arrangements are possible, but not shown. The
carbon in the C-O has only six electrons and violates the
octet rule. A pair of electrons from neighboring oxygen
can be moved over and shared with that carbon. |
 |
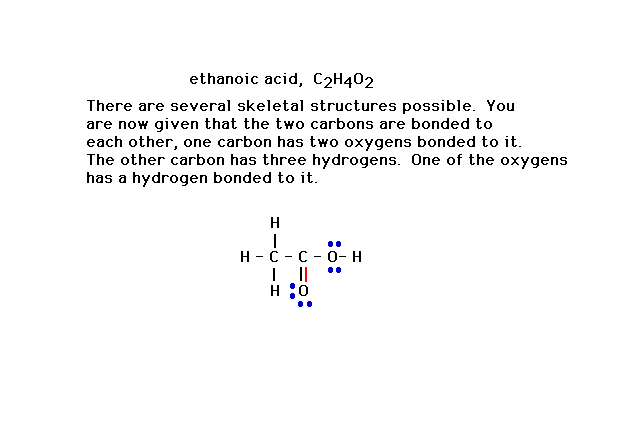
| This is the correct Lewis structure for the compound
identified. (Alternatively, a pair of electrons from the
other oxygen would have satisfied the octet rule as well,
but another consideration -- formal charge -- would
reject that possibility as less favorable. We discuss
formal charge later in the lecture. |
 |
| A complete sequence of analysis would follow steps
you are now at least moderately familiar with. A few
topics relating to further details about structure need
to be addressed next. |
 |
| "Formal charge" for our purposes (in
this course) are to be considered a required part of a
complete Lewis structure. |
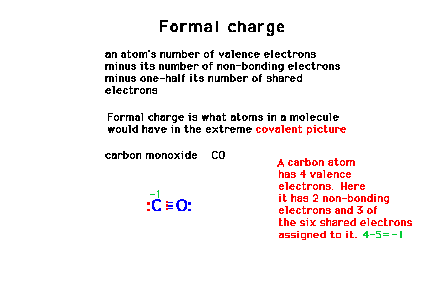 |
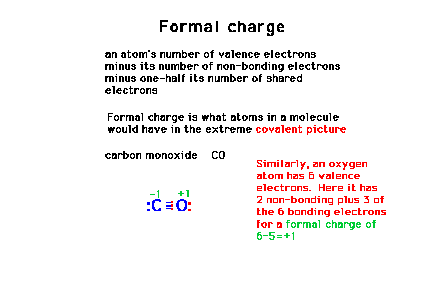
| Calculating formal charge using carbon monoxide as a
simple example. |
 |
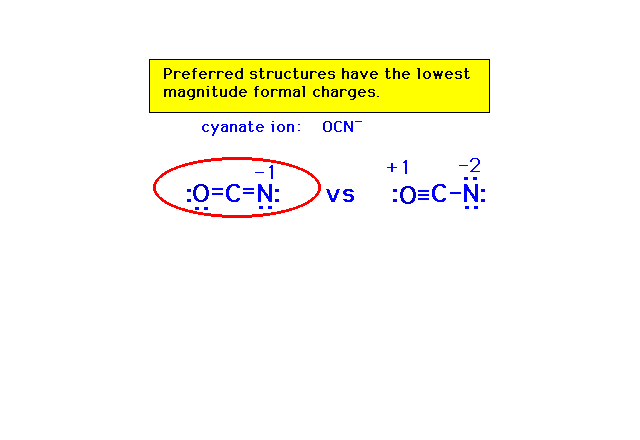
| In deciding among various arrangements of valence
electrons, formal charges serve to indicate which
arrangement is probably closest to the best choice. Such
an arrangement will be referred to as a "preferred
structure." |
 |
| An additional consideration for deciding upon a
preferred Lewis structure in which there are formal
charges. |
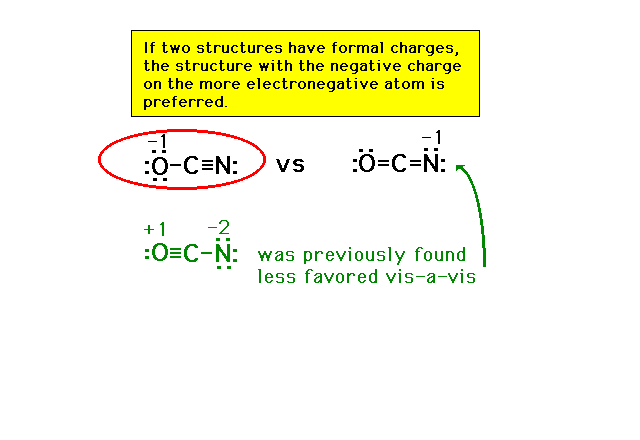 |
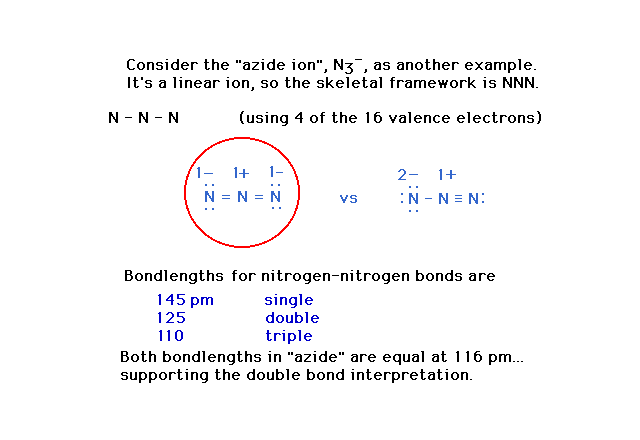
| Using the "azide" ion as another
illustration of deciding upon a preferred Lewis
structure. |
 |


