pH Titration: Answer Sheet Example
- Example Problem:
You are given 100 mL of a "Good" buffer of unknown concentration.
You have a 1.0 M NaOH solution to use in a titration experiment.
- a) Determine the pKa and concentration of the buffer.
- b) Submit your graph of pH vs. equivalents of NaOH.
- c) Identify the "Good" buffer from the list in Campbell, Table 2.7.
- What we know from the problem:
- 1) Volume of the buffer = 100 mL.
- 2) [NaOH] = 1.0 M.
- What we found in the experiment:
|
Vol. NaOH (mL) |
pH |
mequiv.* |
0
0.5
1.0
2.0
3.0
4.0
5.0
6.0
7.0
8.0
9.0
9.5
9.9
10.0
|
4.274
6.31
6.61
6.95
7.19
7.39
7.56
7.75
7.93
8.16
8.46
8.81
9.59
"too large"
|
0.0
0.5
1.0
2.0
3.0
4.0
5.0
6.0
7.0
8.0
9.0
9.5
9.9
10.0
|
|
*Equivalents of NaOH = Volume ¥ concentration.
mequiv. = mL ¥ 1.0 M (i.e. mequiv. = 10-3 ¥ equivalents.)
|
|
Graph of the calculated results:
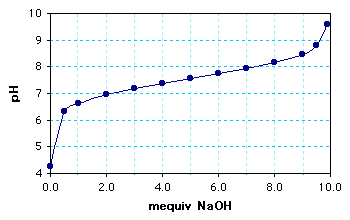
The buffer concentration = 10 mequiv/100 mL = 100 mM or 0.10 M.
The pKa = 7.5-7.6. (The pH at 5.0 mequiv.)
From Table 2.7 of Campbell, the "Good" buffer is either HEPES or TES.
 HEPES.xls is the Excel spreadsheet used to calculate and graph these results; it can be copied to your disk. HEPES.xls is the Excel spreadsheet used to calculate and graph these results; it can be copied to your disk.
|
