Enzyme Kinetics: Answer Sheet Example
| The enzyme concentration = (4 mg/l)/(40,000 g/mol) = 0.10mM (10-7 M). The last two columns were calculated using equations from the Enzyme Kinetics page. |
Graph of the calculated results: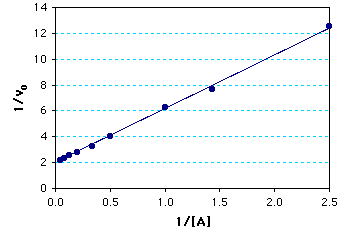 The Km = 2.0 mM; Vmax = 0.5 mM/min. The kcat = Vmax/[enzyme] = (0.5 mM/min)/(0.10mM) = 5¥103 min-1.
|