Enzyme Inhibition Kinetics
- The Michaelis-Menten equation with a competitive inhibitor present is:
-
vo = Vmax[S]/(aKM + [S] )
, where
- vo = the initial velocity;
- Vmax = the maximal velocity;
- KM = the Michaelis constant;
- a = (1 + [ I ]/KI), and KI is the dissociation constant of the inhibitor, I.
- Thus, competitive inhibitors increase KM by a factor of (1 + [ I ]/KI).
- For simple noncompetitive inhibition:
-
vo = Vmax[S]/(aKM + a[S] )
- Noncompetitive inhibitors increase KM, and decrease Vmax by a factor of (1 + [ I ]/KI).
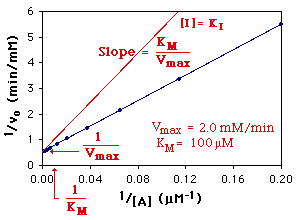
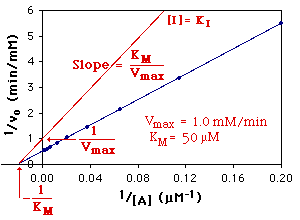
The Michaelis-Menten parameters are determined from the slope and the intercepts of a Lineweaver-Burk Plot as shown below:
(The parameters in red text have been multiplied by 1 + [ I ]/KI.)
Competitive Inhibition:
Noncompetitive Inhibition:
12.2.99


