5.01 Unlike most other substances, water is less dense in its solid phase than in its liquid phase. Also, the density of the liquid phase of water is greatest at approximately 4oC.
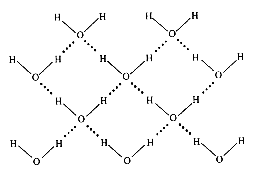
One explanation for the unusual density of water is that ordered clusters of water molecules exist in ice and in the liquid phase below 4oC. When the ordered structure of ice is broken, water molecules may move closer together, filling in the gaps that are present in ice. The density of water will therefore increase as the temperature is raised from 0oC to 4oC. The density of water decreases at temperatures above 4oC. A representation of the structure of ice is shown in Fig 1.

Fig 1
Water in lakes, streams, and oceans contains a variety of impurities and therefore will rarely freeze at 0oC or boil at 100oC. This behavior is due to the freezing-point depression (Kf = 1.86oC/m) and boiling-point elevation (Kb = 0.52oC/m) of water.
The freezing-point depression of seawater may be explained by examining the vapor pressure of seawater as compared to pure, deionized water. Fig 2 shows the vapor pressures plotted against temperature.
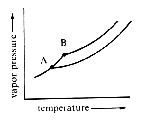
Fig 2
In Fig 2, Point B is the freezing point of pure, deionized water; Point A represents the freezing point of seawater. When seawater freezes slowly, its vapor pressure is the same as that of pure ice at that temperature. This indicates that the ice that freezes from seawater is free of the impurities in the water. However, the concentration of the impurities in the ice depends on the rate of freezing.
| Density
of seawater: Heat capacity of water: Heat of fusion of water: Molalities of the 5 ions in the greatest abundance in seawater: |
1.025
g/mL 75.3 J/mol K 6.020 kJ/mol Na+ (0.470 m) K+ (0.010 m) Ca2+ (0.010 m) Mg2+ (0.054 m) Cl- (0.598 m) |
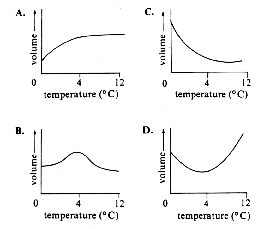
If the temperature of liquid water is increased from 0oC to 12oC, which of the following graphs depicts the relationship between the volume and the temperature of the water?
Ice that slowly forms from seawater will have a sodium ion concentration:
A: close to zero
B: greater than that of seawater
C: equal to that of seawater
D: less than that of pure water
The density of water will decrease as the temperature is raised past 4oC due to which of the following properties of water?
A: freezing-point depression
B: hydrogen bonding
C: thermal expansion
D: dissociation into H+ and OH-
Based on the information given in the passage, which will have a higher boiling point: 1 m glucose or seawater?
A: Seawater, because the total molality of seawater is greater than 1 m glucose
B: Seawater, because glucose has a larger molecular weight than the ions in seawater
C: 1 m glucose, because glucose solutions are not ionic
D: 1 m glucose, because the total molality of 1 m glucose is less than seawater
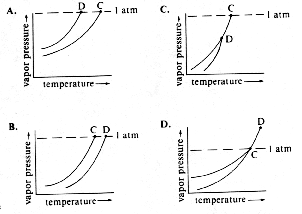
Which of the following graphs best represents a plot of the vapor pressure of seawater and pure water near their respective boiling points? (Note: Point C is the boiling point of pure water; Point D is the boiling point of seawater.)
Based only on the information in the passage, which of the following expressions should be used to best estimate the temperature (oC) at which ice will form in seawater?
A: 0 - 1.86(6.020)
B: 0 - 1.86(1.025)
C: 0 - 1.86(4.184)
D: 0 - 1.86(1.142)