Supplementary Problems
1. Rank the following substances in order of increasing solubility in water (and understand the reasons behind the choices): C6 H6 (benzene), HOCH2 CH(OH)CH2 (OH) (glycerine), and C5 H11 OH (pentanol).
2. Water is not the only substance used as a solvent. Rank the same three substances from the above problem in order of increasing solubility in CS2 (carbon disulfide, whose structure you might want to propose first).
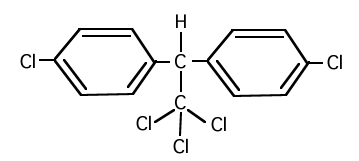
3. Dichlorodiphenyltrichloroethane (DDT) has a structure shown below. Its dipole moment is just slightly larger than 1 D. Will this substance concentrate in fatty tissue or in aqueous intracellular fluid in living systems?

4. Acetone, (CH3 )2CO, is miscible in both water and cyclohexane, C6 H12 . What change in solubility should you expect for thioacetone, (CH3 )2 CS?