Department of Chemistry
CARNEGIE MELLON UNIVERSITY
09-105 Introduction to Modern Chemistry
Quiz #2 Name
Closed book, 10 minutes January 28, 1999
Which of the following combinations of quantum numbers are allowed for an electron in a one-electron system and which are not?
(a) n = 3, l =3, m = 1, ms = 1/2
(b) n = 3, l = 3, m = 0, ms = -1/2
(c) n = 5, l = 1, m = 2, ms = 1/2
(d) n = 4, l = -1, m = 0, ms = 1/2
For n = 1 and l = 0, the orbital would be labeled a "1s" orbital. Label the orbitals described by each of the following sets of quantum numbers.
(e) n = 4, l = 2
(f) n = 6, l = 4
(g) n = 3, l = 1
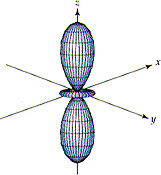
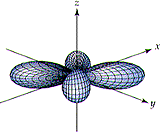
Which of the figures below, A, B, or none, could be correctly used to describe a
(h) 3pz orbital
(i) 3dx2-y2 orbital
(j) 6dxy
orbital
 |
 |
| (A) | (B) |