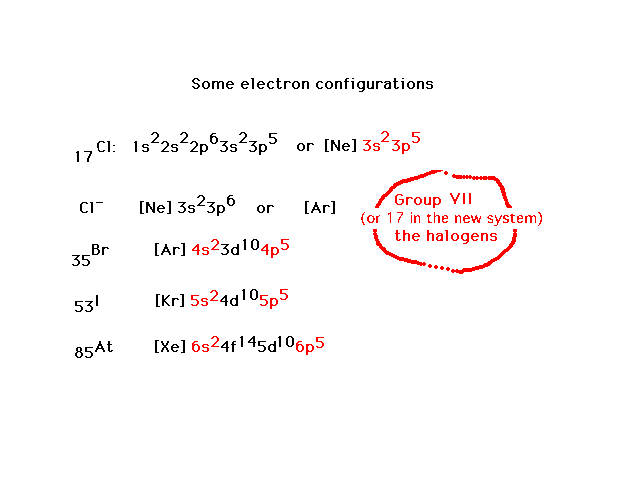| Lecture
#7 |
| Text sections: 12.13, 14, 15 |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
The Periodic Table (continued)
Sizes
Isoelectronic species
Ionization energies
|
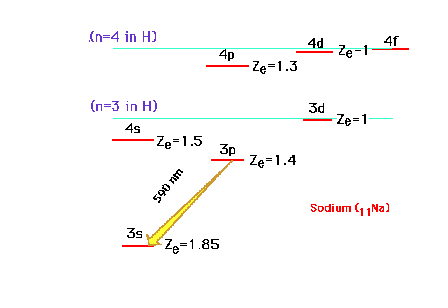
| Continuing our discussion of effective
nuclear charge, we move on to the sodium atom with Z=11.
The electron configuration is 1s22s22p63s1
or [10Ne]3s1. The valence electron
is the 3s electron, which can be promoted to the excited
states illustrated. Note that because of the great
penetrating probability of an electron in the s-orbital,
the 4s becomes lower in energy than the 3d orbital. The
yellow (590 nm) emission line of an excited Na is
indicated. |
 |
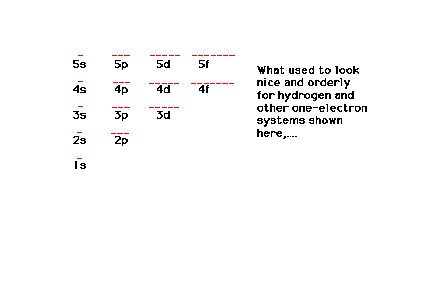
| A schematic view of the arrangement of
orbitals for one-electron systems |
 |
| A schematic view of the arrangement of
orbitals for many-electron systems |
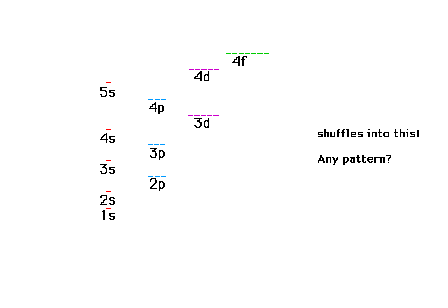 |
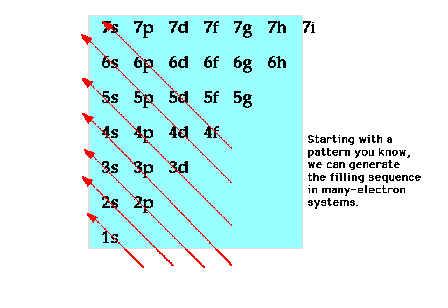
| A mnemonic device for reproducing the
order of orbitals according to increasing energy for
many-electron systems. |
 |
| Here are configurations for four atoms (and one ion).
Each atom is characterized now by the number of s and p
valence electrons...7...accounting for the similar
properties of these "halogen" elements in their
chemistry. |
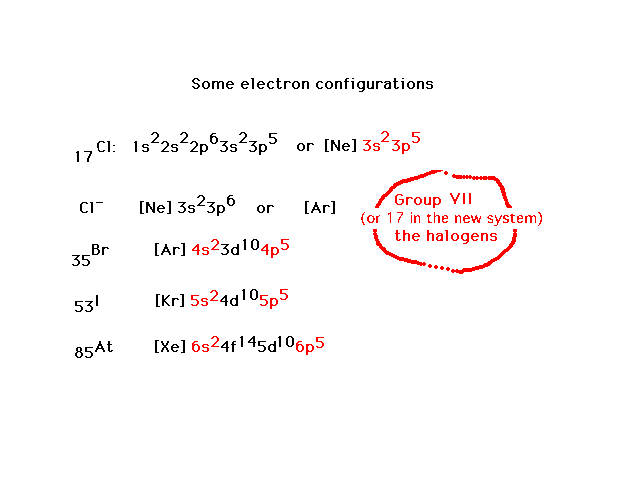 |
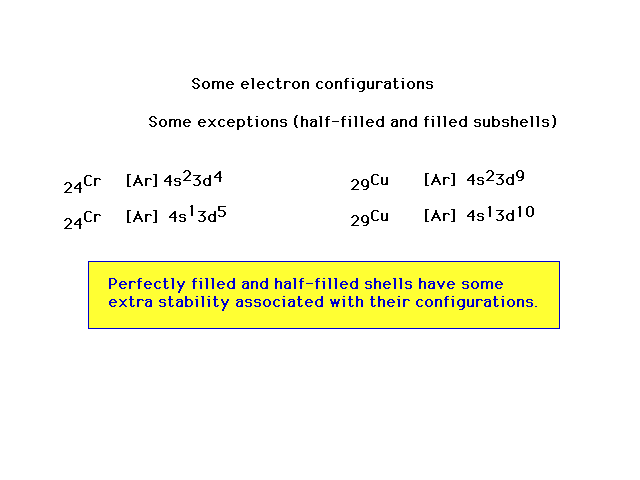
| Exceptions appear in the filling scheme owing to an
"extra stability" associated with completely
filled subshells and half-filled subshells. |
 |
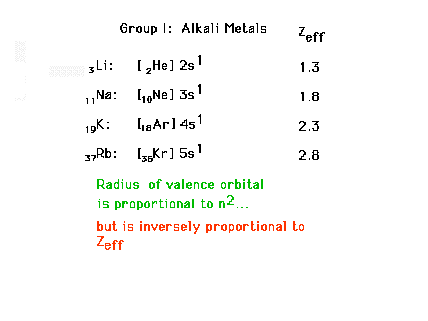
| Electron configurations for Group I elements, the
alkali metals. Sizes are determined by "n" and
Zeff for the last electron. |
 |
| The trend in radii for the alkali elements |
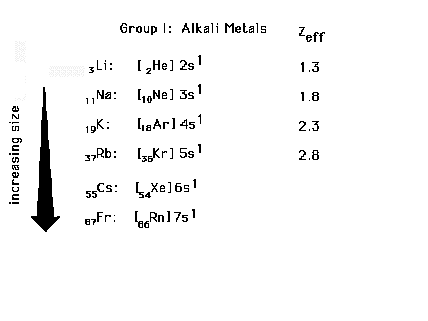 |
| Generalization of the trend in radii for a Group
(column) in the Periodic Table |
 |
| The trend across a row of the Periodic Table, where
"n" is fixed for the main groups, is determined
by Zeff, which increases. (Main groups are the
s-block and the p-block elements.) |
 |
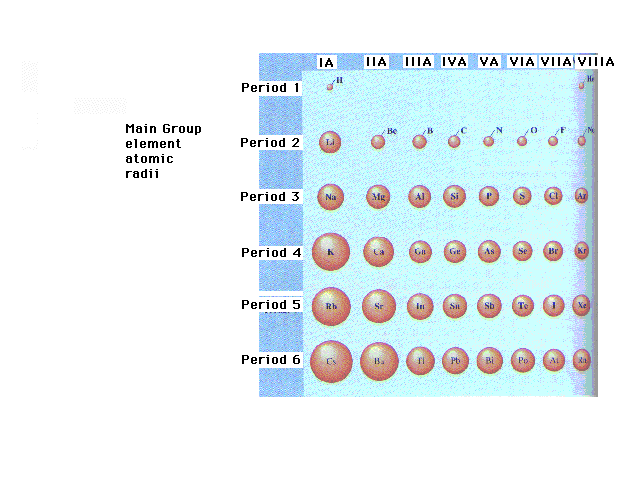
| The general trend across main group rows is a
decrease in size from left to right in the Periodic Table |
 |
| The trends for "Main Group"
("Representative" or "s- and
p-block") Elements. |
 |
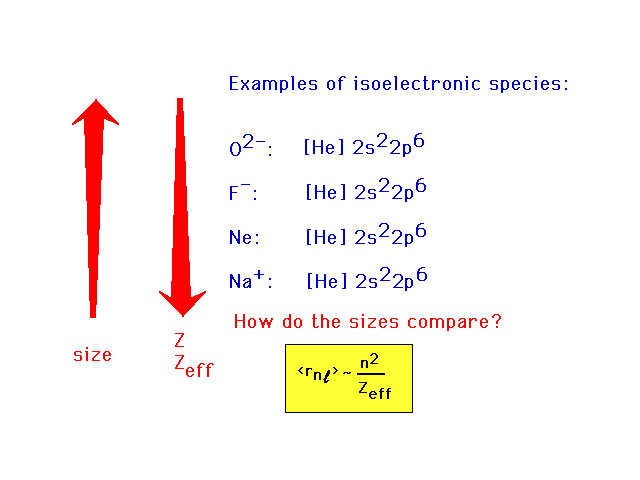
| Isoelectronic systems have the same electron
arrangements. The example here can be generalized to any
similar sequence. |
 |
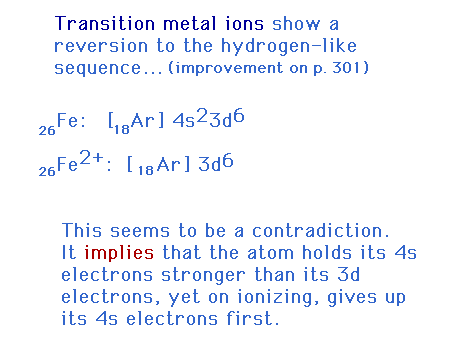
| All the rules we've seen about the electron
configuration filling sequence apply to both atoms and
ions of the main group elements (s-block and p-block) and
to the transition metal atoms. For transition metal ions,
the sequence changes! |
 |
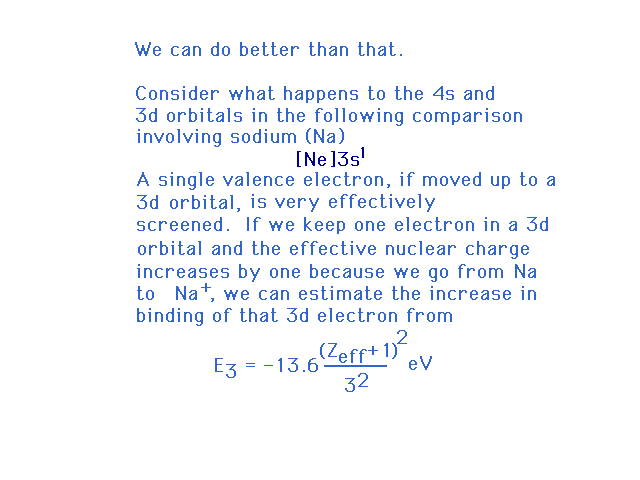
| To explain why the 4s-3d order shifts when
ionizing an element, we resort to a familiar example,
sodium, and look at its (excited) 4s and 3d excited
one-electron orbital energies. Zeff refers to
that for atomic Na in this illustration |
 |
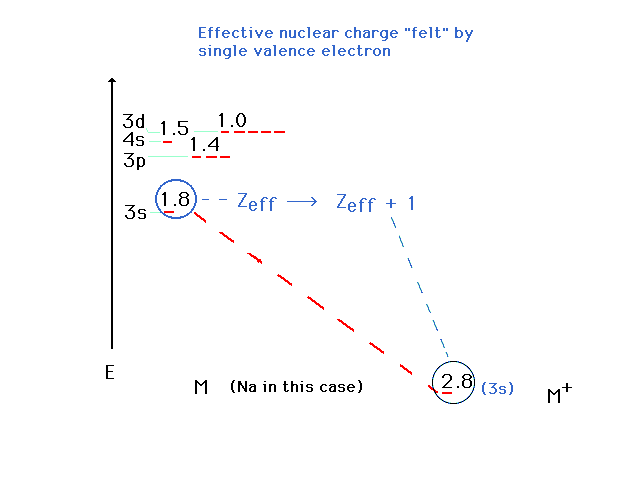
| The states are graphed at their proper
energies, but we've indicated the effective nuclear
charges drived from these energies. If an inner electron
is removed completely from Na, we can estimate that the Zeff
for the outer valence electron osup by approximately 1. |
 |
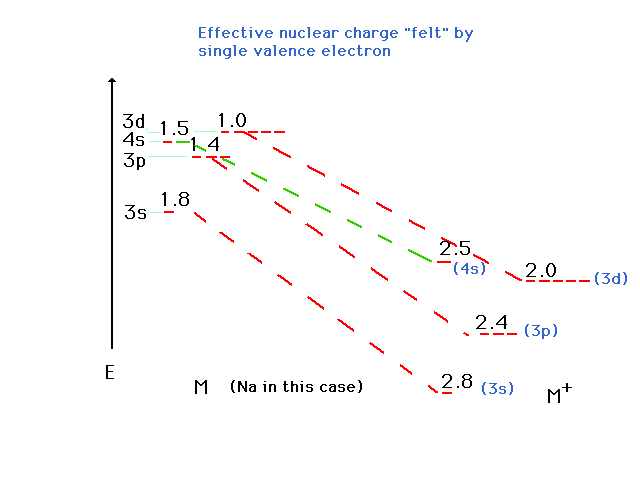
| The other changes are similarly estimated and
-- lo and behold -- the 3d is now more stable than the 4s
orbital. When these are filled as in the transition metal
ions, the 3d is occupied while the 4s is empty. (Keep
in mind that the values shown are just the effective
nuclear charges, but the position of the energy level
corresponds to E as determined by n and Zeff.)
|
 |
| For this course, 09-105, at CMU, we will adopt the
general rule that notes a different configuration
decision about transition metal ions as
opposed to atoms and main group ions. |
 |