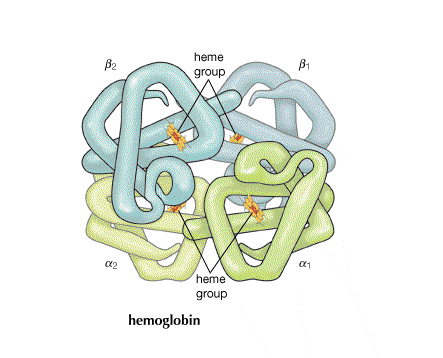
Hemoglobin
Bonding of heme Fe2+ to O2 vs CO
Sizes of transition metal ions
Role of protein in hemoglobin
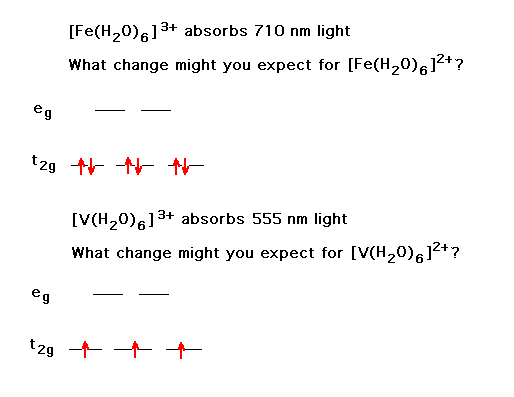
| Lecture #34 | |
| Hemoglobin...an accumulation of all the chemistry we have seen in this semester course. | CURMUDGEON GENERAL'S WARNING. These "slides" represent highlights from lecture and are neither complete nor meant to replace lecture. It is advised not to use these as a reliable means to replace missed lecture material. Do so at risk to healthy academic performance in 09-105. |
| Lecture Outline | Transition Metal Complexes Hemoglobin
|
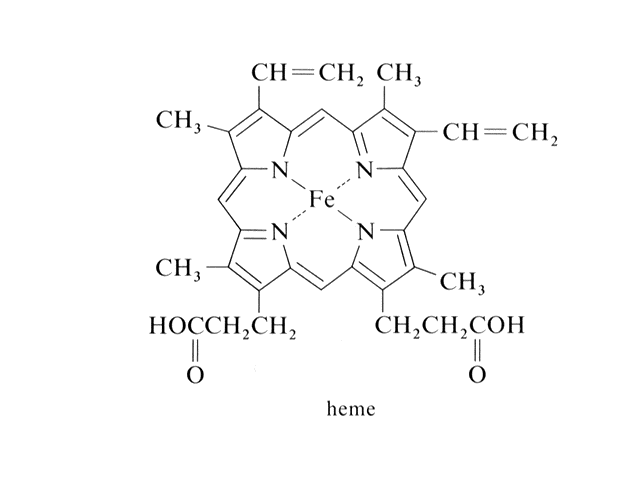
| "Heme" is where the discussion of transion metal ion complexes started. Here is the Fe2+ in a square planar, coordination number four complex. |  |
| In hemoglobin, there are four hemes bonded to the large protein structure with molecular weight about 68000. |  |
| The chemistry of oxygen in hemoglobin starts with a look at the Fe2+ at the heme site. | |
| The chemistry of oxygen in hemoglobin starts with a look at the Fe2+ at the heme site. | |
| In the absence of oxygen at the sixth octahedral site, the geometry of the complex ion changes and this geometric shift is mechanically transmitted to the other heme sites on the hemoglobin molecule making it easier for those to give up their oxygens as well. This leads to cooperativity among sites for both oxygen release (at cells being fed oxygen) and also oxygen takeup (by red blood cells in the lungs). Why the geometry changes has to do with the ability of a small ion to fit in the planar coordination site and a large ion to be pushed out. | |
| The effects of adding electrons across the transition metal ion series produces some surprises, although they're understandable, showing the influence of the complexes' geometries and ligand character. This is the behavior of ionic radii within a complex ion for strong field octahedral ligands. | |
| The counterpart to the above is the change in the trend when weak field ligands are considered. | |
| Changing a transition metal ion's oxidation state has some understandable consequences. Here, we look at the shif in color. | 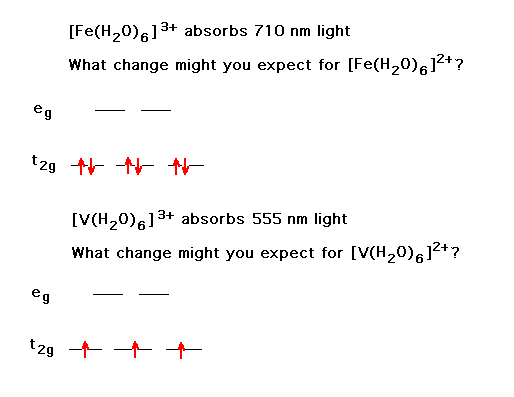 |
| Atomic orbitals in the iron ion. | |
| The d-orbital configuration in a strong field complex. | |
| The CO is normally bonded in a linear fashion with the Fe2+ transition metal ion. | |
| The oxygen molecule is bonded through a bent, non-linear bond with the Fe2+. | |
| A reminder of the electron states available to valence electrons in CO. Note the empty pi antibonding orbitals (whose shapes you should remember). | |
| The dxz is present too. It contains a pair of electrons (from the iron). | |
| The dxz orbital from the iron and the pi antibonding orbital from the CO overlap effectively and give rise to a delocalized orbital involving Fe, C and O. Yes, you're feeding electrons into the carbon-oxygen bond antibonding state which would weaken the CO bond, but you are also strengthening the Fe ligand bond by producing some pi bond character there. Thus, CO bonds very strongly to iron (and CO is a strong field ligand). | |
| Where have we gotten in our discussion of bonding to iron in heme? CO is expected to completely tie up all the iron sites in heme because of the stronger bond than produced with oxygen binding there. Why are we alive? | |
| One explanation is that when the heme is in its protein, part of the hemoglobin structure forms a canopy over the oxygen binding site that geometrically prevents carbon monoxide from binding in a linear fashion. The pi bond character is eliminated and even the remaining sigma bond is not too effective, thus allowing oxygen and carbon monoxide to compete for iron on a more level playing field. | |
| The side chains influence how the protein affects chemistry. Non-polar side groups at the entry to the Fe-heme keep polar species (water, cyanide) away. Oxygen is non-polar. Carbon monoxide, somewhat surprisingly, has only 2% ionic character and is therefore basically also non-polar. | |
| The capture and release of H+ by hemoglobin when oxygen is released to "burn" glucose or picked up from the air in lungs, respectively, facilitates the release of CO2 from dissolved bicarbonate and removal of CO2 waste into blood plasma at the glucose oxidation sites. | |