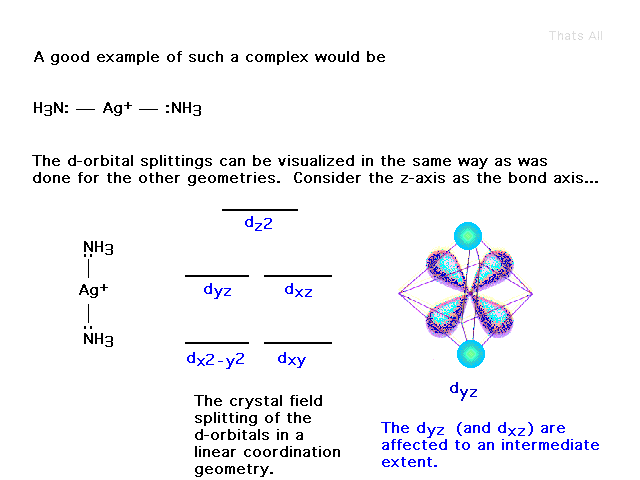| Lecture
#35 |
| Review for Exam IV |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| The list from which questions will be drawn for Exam
IV on Weds, Dec 1. |
 |
| ...topics, topics, |
 |
| A review problem for stimulating thought
and discussion. It will
take several steps to work through. It is much too long
and complex to worry about as a possible exam question. |
 |
| |
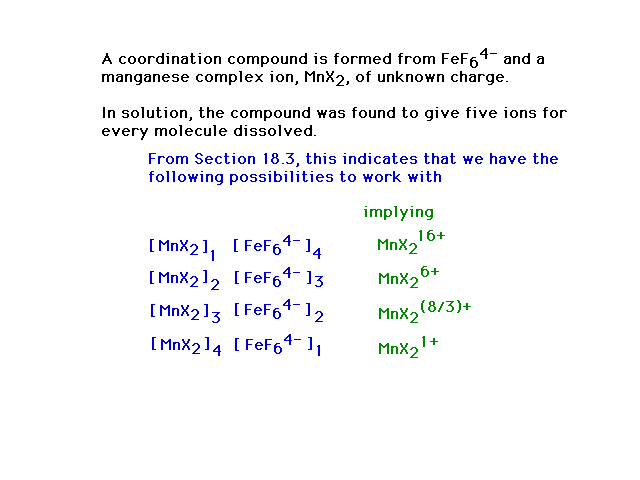 |
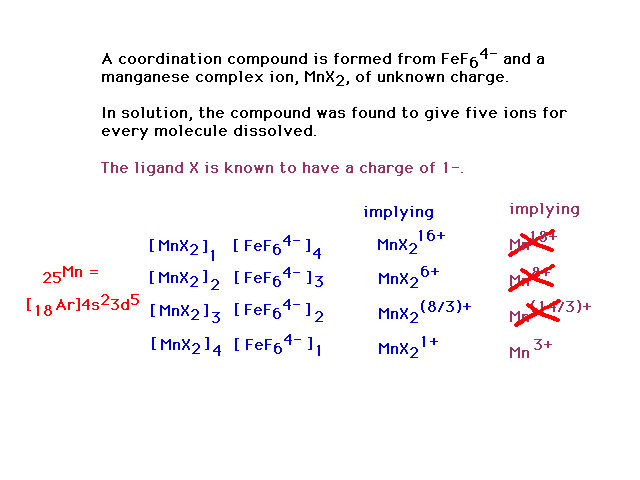
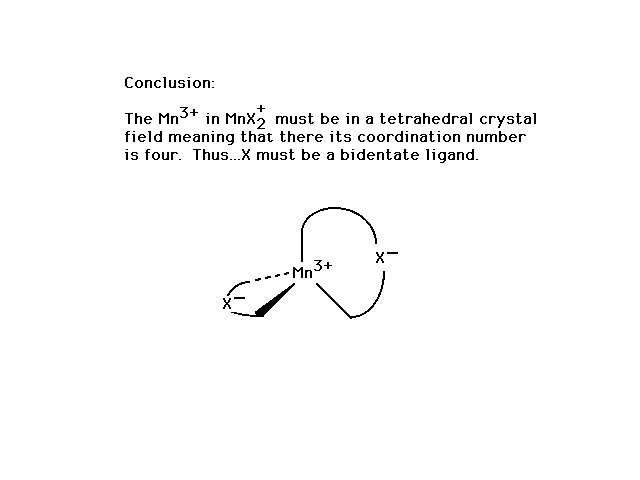
| From the position of Mn on the periodic table and
understanding of oxidation numbers, the possibilities are
+7 through 0, ruling out all but +3. |
 |
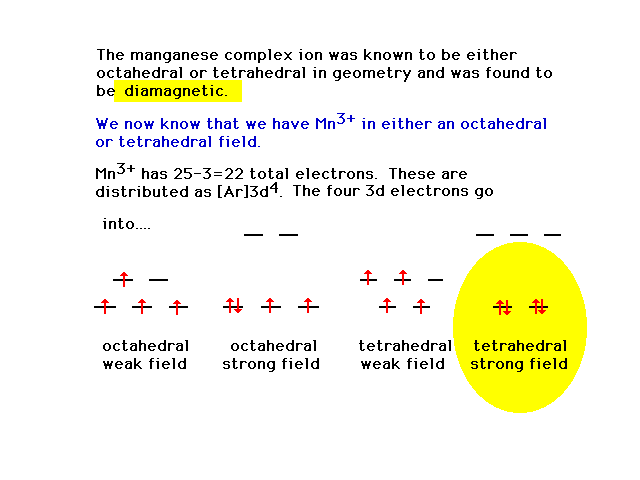
| Two geometries and the extreme weak/strong field
arrangements in combination with the given magnetic
property allow the illustrated conclusion (highlighted). |
 |
| |
 |
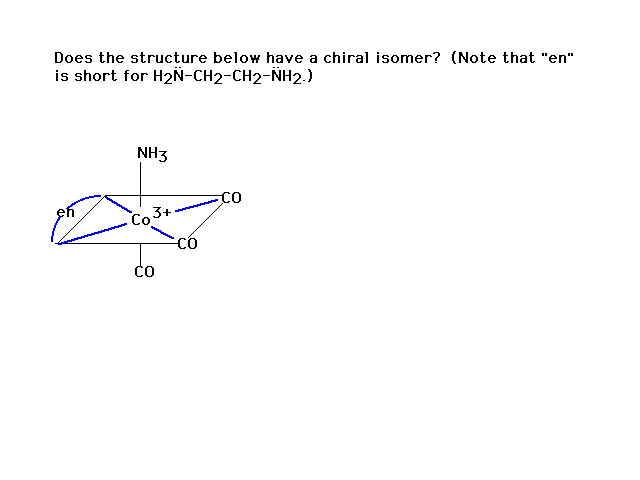
| Another practice problem |
 |
| |
|
| And another |
 |
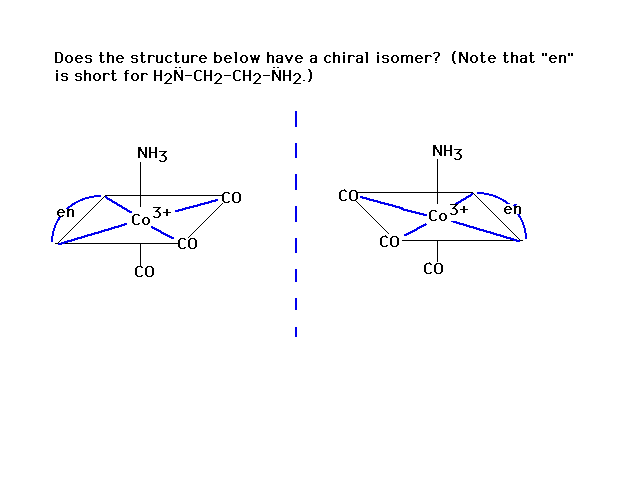
| This is superimposable on the original geometry. Not
a chiral isomer! |
 |
| ..on and on we go.... |
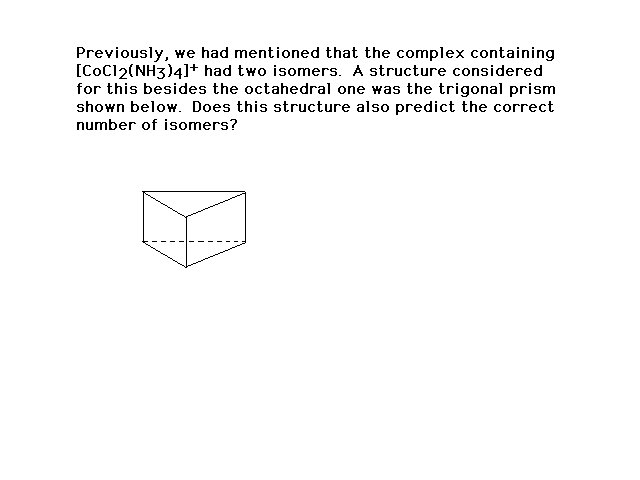 |
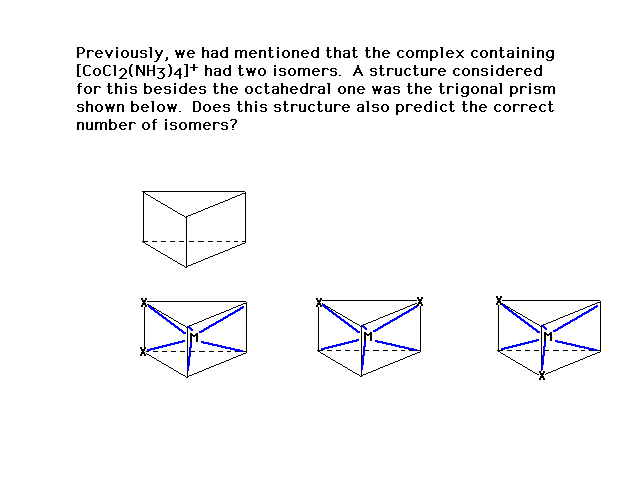
| If this were the correct geometry for the ligands,
three geometric isomers would have been found. Since
"two" is the given result, this cannot be the
correct arrangement. |
 |
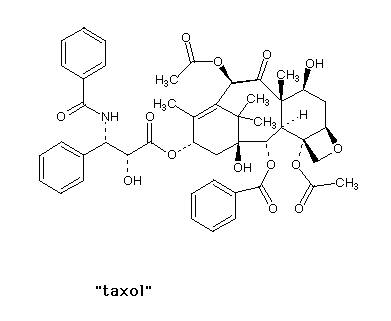
| If each asymmetric carbon gives you two isomers
(enantiomers/chiral isomers), then two such carbons in
the same molecule gives you four possible isomers. How
many isomers of taxol are possible? There are 11
asymmetric centers giving 2048 total isomers.
|
 |
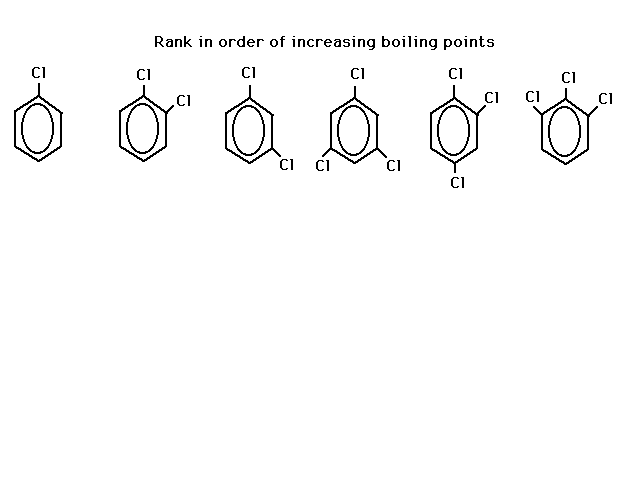
| Rank the following six chlorobenzenes in order of
expected increasing boiling points. |
 |
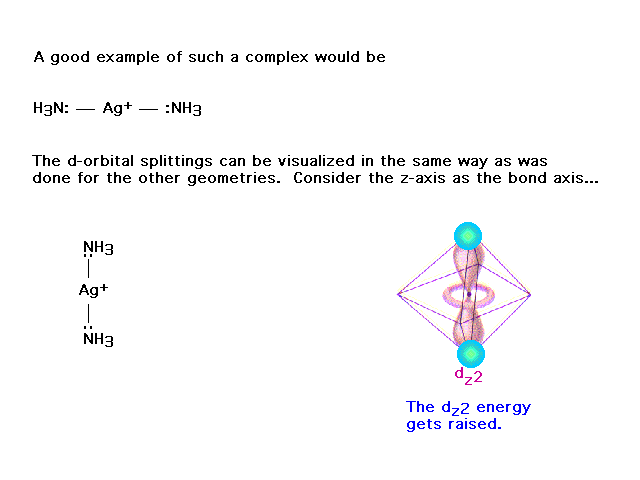
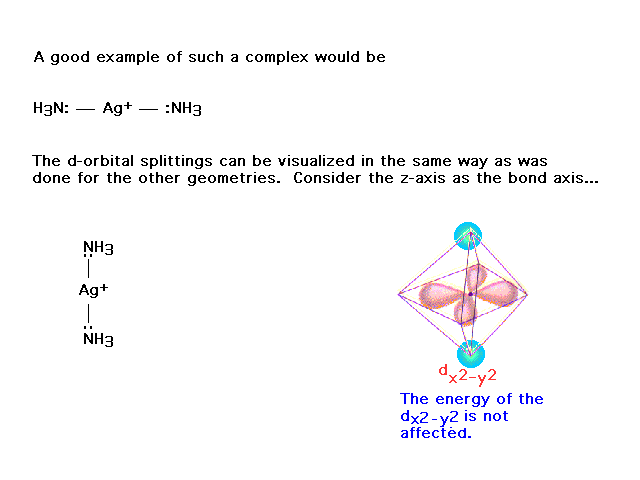
| (This was not included from an earlier lecture.)
Here's another attempt to show how crystal field
splitting works, but with coordination number 2, also
known as linear geometry. |
 |
| This orbital's electron, pointing directly at the
ligands (with their electrons) is raised in energy. |
 |
| On the other hand, this orbital does not point
towards the ligands and is unaffected by their presence.
Likewise for the dxy. |
 |
| Putting it all together, the crystal field idea
rearranges the five d-orbitals into the illustrated
diagram. |
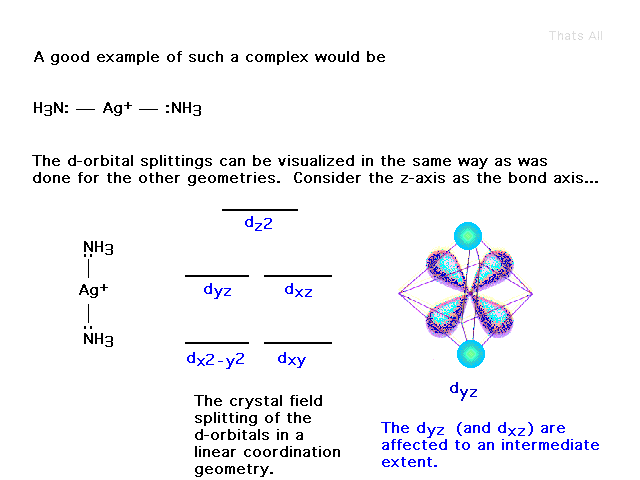 |