| Lecture
#21 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Delocalized molecular orbitals
- ozone
- Particle-in-a-box model for energy states
|
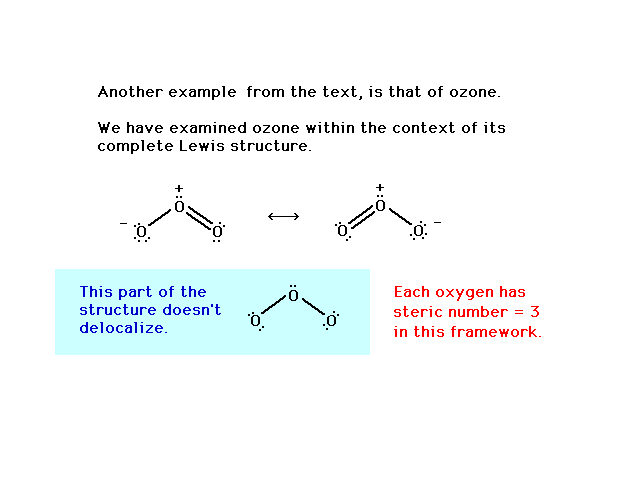
| The ozone molecule's Lewis structure shows that even
the preferred structure in this case indicates resonance
is required for a complete description of the molecule's
valence electrons. Note that a pair of electrons involved
in bonding oxygens is delocalized. But also note that a
"non-bonding", lone pair is also resonating
between both end sites in the Lewis structure. |
 |
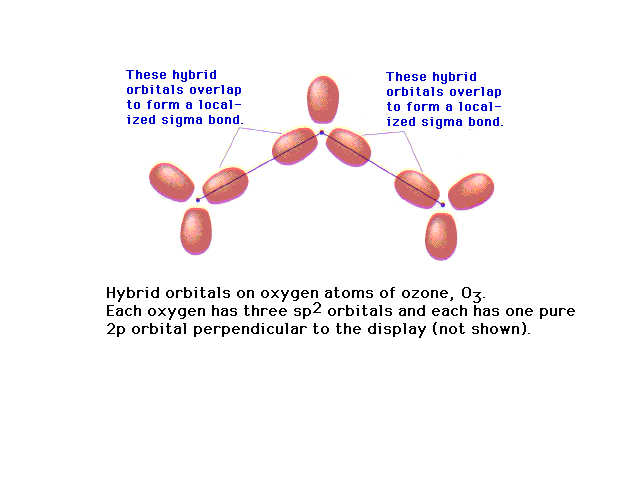
| Of the 18 valence electrons in ozone, two pair are
involved in resonance. The remaining 14 electrons
constitute the sigma bonded framework shown here as
coming from sp2 hybrids at each oxygen (since
each is in an electronic geometry involving three
groups). |
 |
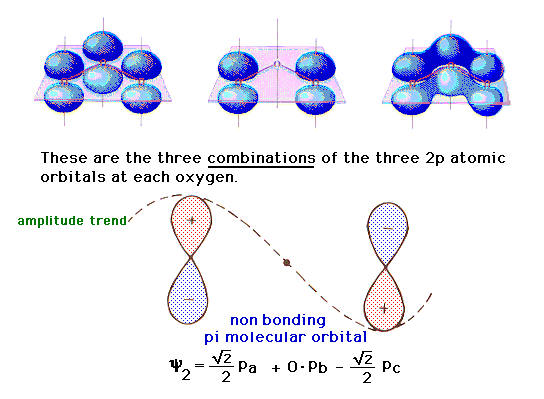
| There is a pure 2p atomic orbital at each oxygen
perpendicular to the plane of the molecule. These lead,
via linear combinations, to three delocalized pi
molecular orbitals. The one involving constructive
interference leading to a bonding molecular orbital is
illustrated at the right. |
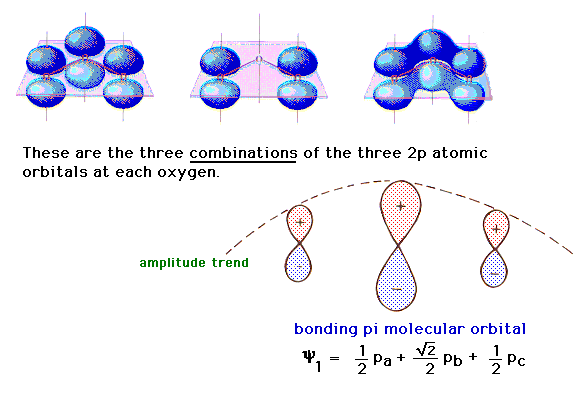 |
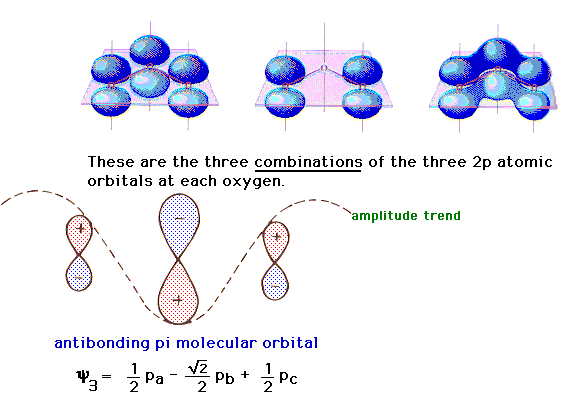
| The linear combination in which the 2p atomic
orbitals consecutively interfere leads to an antibonding
orbital as shown on the left. |
 |
| The final combination involves (for reasons that
emerge in the mathematics of the wave equation treatment)
a "zero weighting" on the middle 2p orbital.
The result is a molecular orbital that is basically
indistinguishable from what looks like two pure 2p atomic
orbitals, one at each end, as shown in the middle. This
is consequently a non-bonding molecular orbital
(because there is neither constructive bonding nor
destructive antibonding between pairs of atoms). |
 |
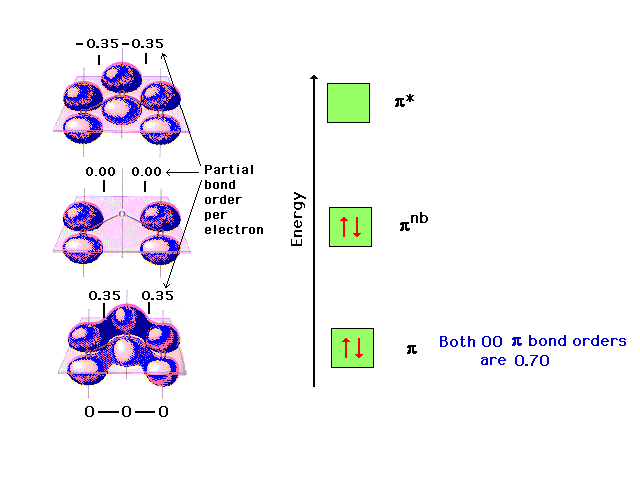
| The pi molecular orbital energy diagram for ozone
into which are distributed four valence electrons. Two
are in the bonding orbital and yield a bond order of 0.5,
which when added to the sigma bond corresponds to a bond
order of 1.5 between each of the oxygens. The remaining
pair is distributed at each end, corresponding to the
negative charge simultaneously at each end oxygen in the
Lewis structure. |
 |
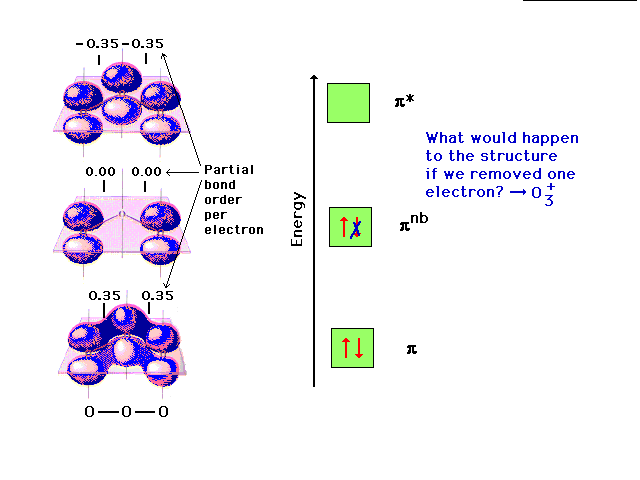
Changing the electron configuration of non-
bonding orbitals does not affect the structure
significcantly. |
 |
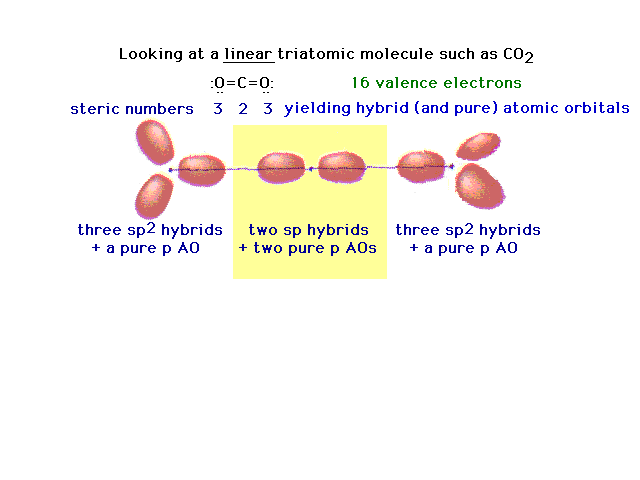
| Another triatomic molecule is carbon dioxide. The
text presentation of this is awkward, so it's completely
re-constructed here following the arguments that should
seem familiar. Here's the underlying framework with the
hybrids on the end oxygens lying in two planes
perpendicular to each other. This arrangement will lead,
in the next slide, to the ready formation of two double
bonds. |
 |
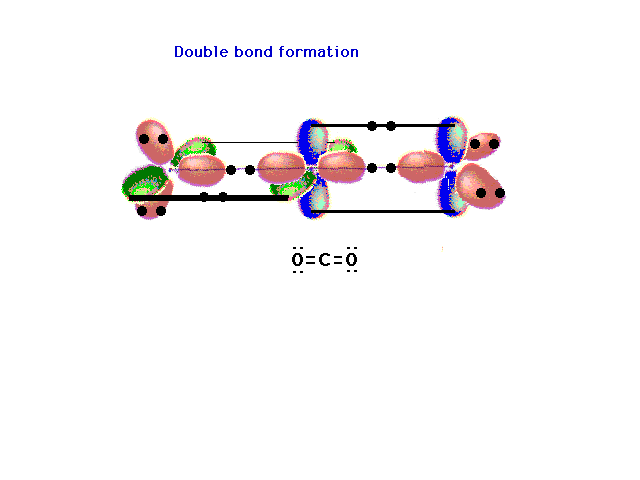
| The pure p orbitals perpendicular to the hybrids
overlap effectively in pairs -- not all three at once --
as shown here. Two pure double bonds arise in this
manner. The placement of all 16 valence electrons is
indicated. |
 |
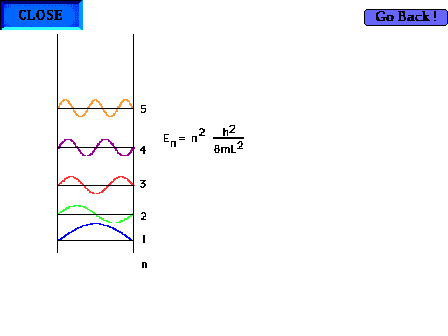
| The particle-in-a-box model can now be profitably
revisited. Showing the first five waves and their
energies. There are, of course, an infinite number of
such possibilites corresponding to shorter and shorter
wavelengths and higher and higher energies. |
 |
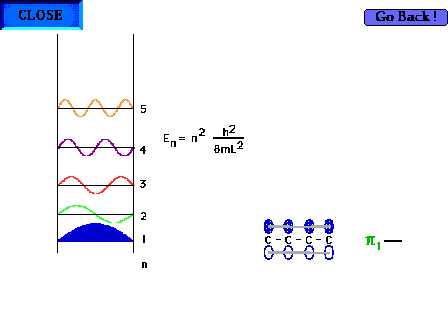
| The shape of the n=1 particle-in-a-box wave function
approximates the appearance of the ground state pi wave
function for the delocalized molecular orbital in 1,3
butadiene for instance. |
 |
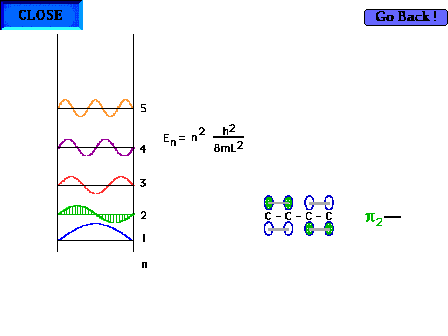
| The shape of the n=2 particle-in-a-box wave function
approximates the appearance of the p2
orbital in a delocalized system. |
 |
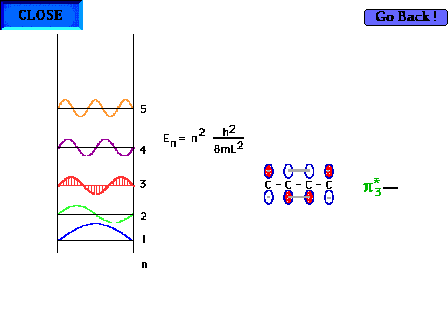
| The shape of the n=3 particle-in-a-box wave function
approximates the appearance of the p*3
delocalized orbital. |
 |
| The shape of the n=4 particle-in-a-box wave function
approximates the appearance of the p*4
delocalized orbital. |
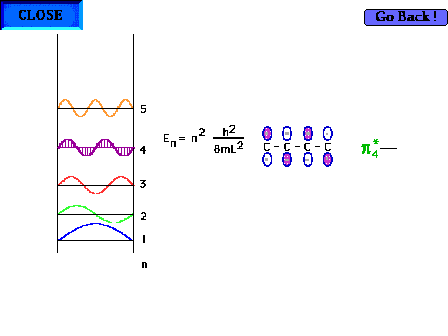 |
| There are four electrons "free" to
delocalize in the 1,3 butadiene system H2C=CH-CH-CH2 |
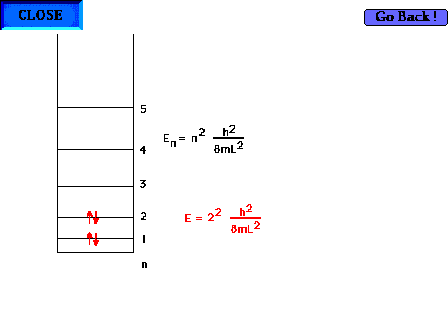 |
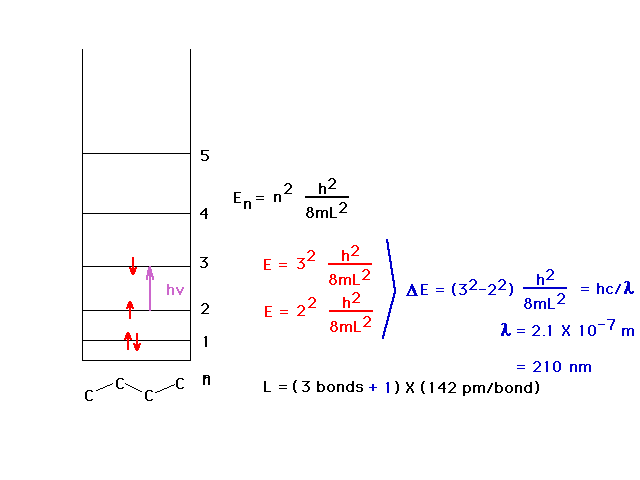
| The average carbon-carbon bondlength (C=C and C-C) is
142 pm and allows calculation of the wavelength of the
absorbed light by using the particle-in-a-box to estimate
the energy levels. The ultraviolet wavelength is 210 nm.
We allow the box to extend beyond the outer nuclei by
another one-half bond at each end. |
 |
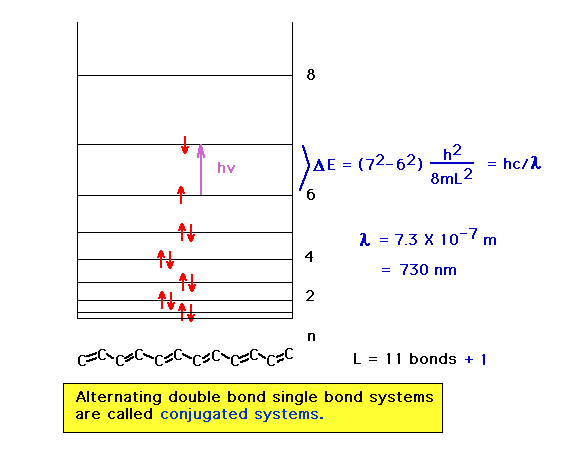
| Here we have a more delocalized system in which the
absorbed wavelength has consequently shifted to the
visible region of the electromagnetic spectrum and the
highest occupied orbital starts out with a larger n
quantum number. |
 |
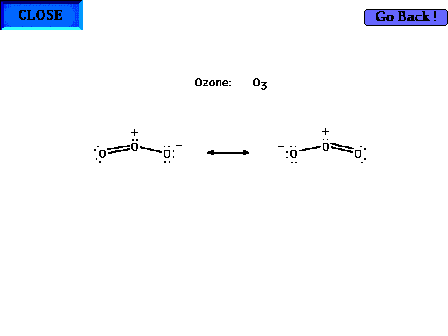
| Another example. Ozone as a delocalized electron
system. |
 |
| The particle-in-a-box model approximation for the
delocalized orbital energies in the ozone molecule shows
that absorption takes place in the ultraviolet region of
the electromagnetic spectrum. From what you have seen
concerning the pi orbitals in ozone, this absorption
corresponds to raising an electron from a non-bonding
orbital to an antibonding orbital. |
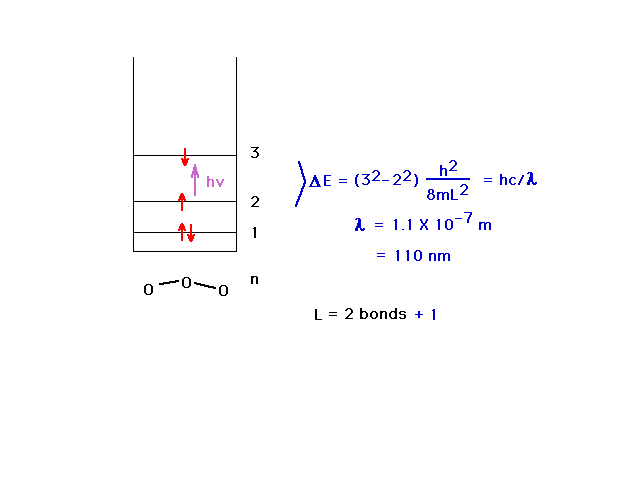 |
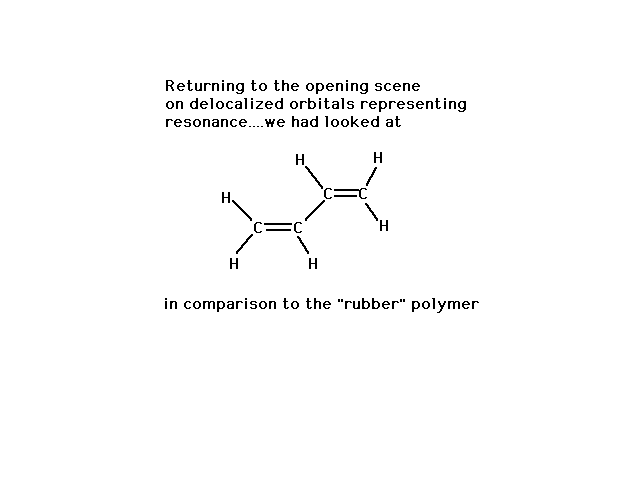
| We began the look at delocalized molecular orbitals
with a discussion of the (energy and geometry) properties
of 1,3 butadiene, indicating that we could understand
them if the central CC bond had some double bond
character. |
 |
| Yet just previously, we noted a similar looking
structure had the flexibility associated with
unrestricted rotation about a sigma bond. |
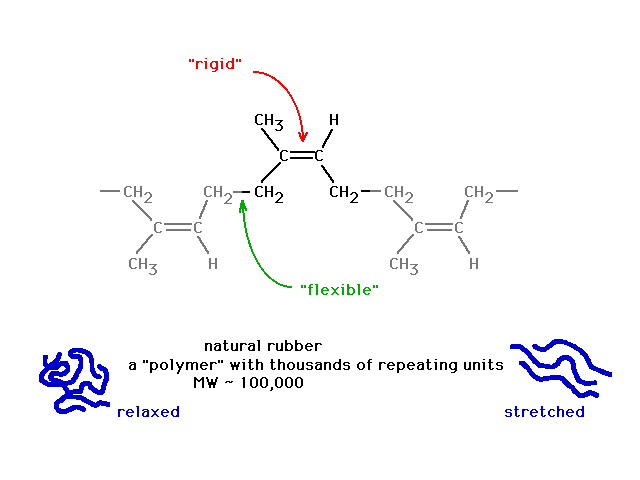 |
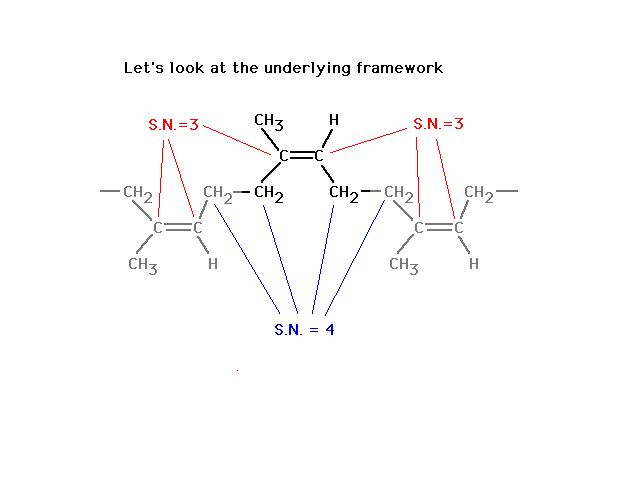
| The difference, reminding us of what leads to
delocalized molecular orbitals, is revealed in looking in
more detail at the situation where we do get unrestricted
rotation, starting here... |
 |
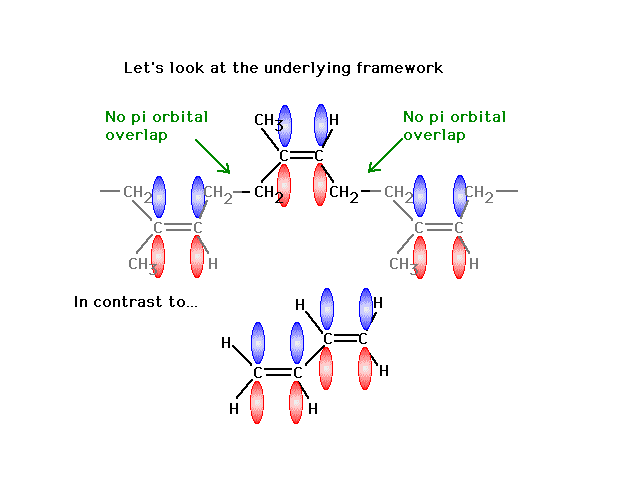
| Finally, a look at the atomic orbitals shows that in
"rubber" one gets localized pi bonds whereas in
the butadiene, delocalized pi bonds arise. |
 |




