| Lecture
#2 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
History (Stoichiometry and
Periodicity)
Early recognition of "Periodicity"
Energy
Kinetic Energy
Potential Energy
Internal Energy
Light
Wavelength, frequency, amplitude, wave number
Energies
H-atom line spectra (emission and absorbtion)
Masses
Mass spectra
|
| Avogadro accepts the significance of Gay-Lussac's
experiments and hypothesizes some significant ideas. |
 |
| Dalton disputes the significance of Gay-Lussac's
results and regards Avogadro's ideas as erroneous. |
 |
| Nearly fifty years go by before order is restored by
Cannizzaro to the "science" of chemistry. |
 |
| Simple measurements on equal volumes of gases in
conjunction with chemical analysis of the composition of
the gases and acceptance of Avogadro's hypotheses leads
to a unique collection of "combining weights"
of the atoms. |
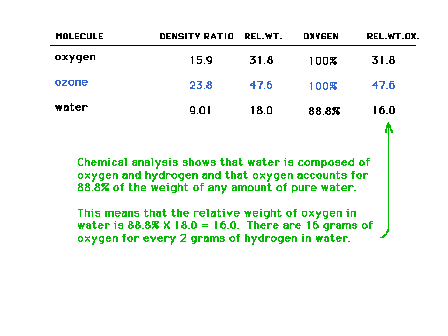 |
| Gas analysis data on oxygen-containing gases. Note
that there are three compounds on this list that contain
only nitrogen and oxygen. The data can be used to
demonstrate the Law of Multiple Proportions. |
 |
| Mendeleev recognizes the periodic behavior of
combining ratios (or "valency") of the atoms of
elements. |
 |
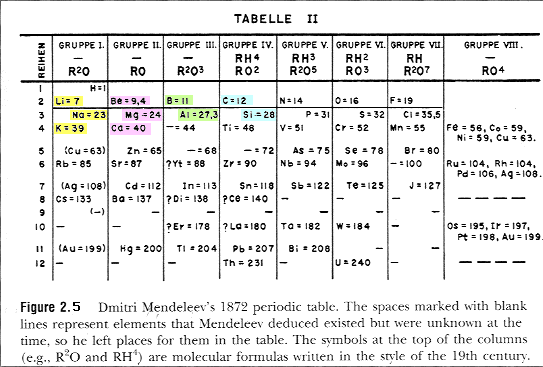
| (This was missing from lecture.) In 1869, the Russian
chemist Mendeleev noted that the repeating patterns of
behavior could be arranged in a sequence of elements
giving rise to the "Periodic Table" of the
elements. |
 |
| We'll be spending some time looking at light and the
energy associated with light. First, let's establish some
terms related to energy and couple of familiar examples
of potential energy. Potential energy is related to
forces acting on a particle. |
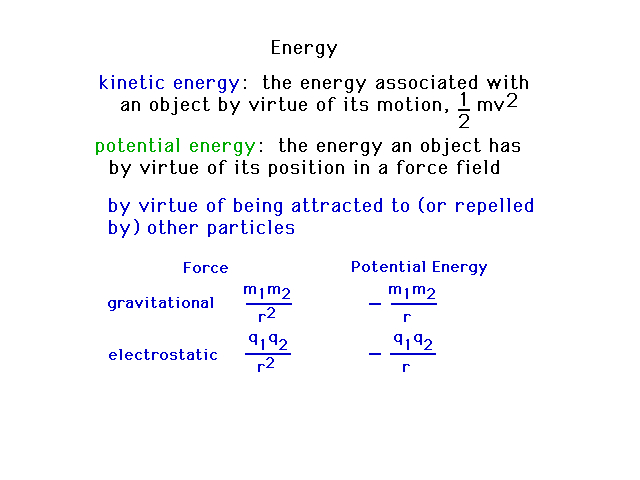 |
| The different types of
energy that we will distinguish in our upcoming
discussions. |
 |
|
 |
| By the end of the 1800s, the nature of light was
moderately well understood. Light has an oscillating
electric and magnetic field. The amplitude of a
"light wave" is shown |
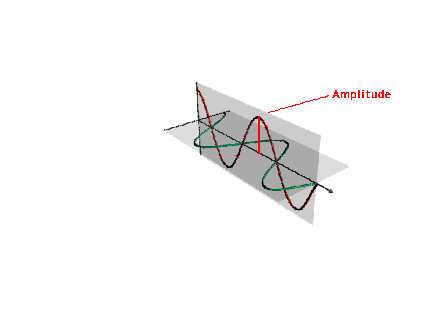 |
| The wavelength of a light wave |
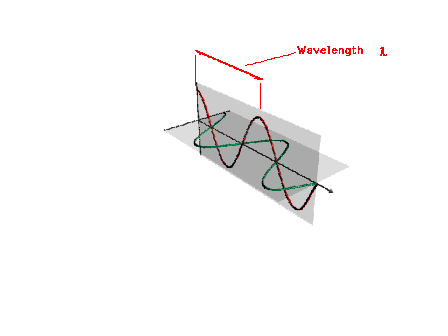 |
| Wavelength, frequency and wave number are related
through the speed of the traveling wave. (Wave number is
not discussed in the text. It will show up in other
courses and is presented here for completeness. You need
not know it for 09-105.) |
 |
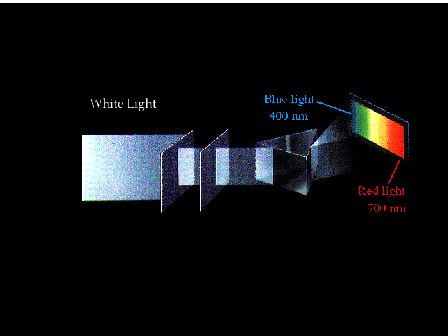
| Dispersion of white light through a prism producing
the familiar rainbow spectrum. You need to know the
wavelengths of blue and red light. |
 |
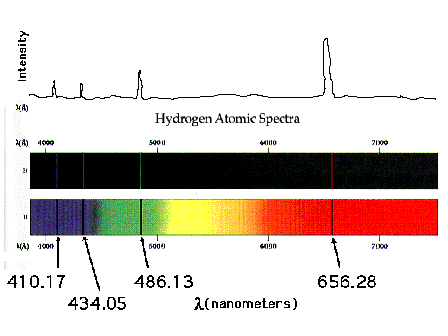
| A serious puzzle involving light was the existence of
line spectra, one example of which is shown here: the
line spectrum of the hydrogen atom. Emissions and
absorbtions of light (energy) occur at very precise
wavelengths (energies).Why? The answer was not known yet. |
 |
| The first fundamental particle is discovered, the
electron. Structure of the atom is about to become a bit
clearer. |
 |
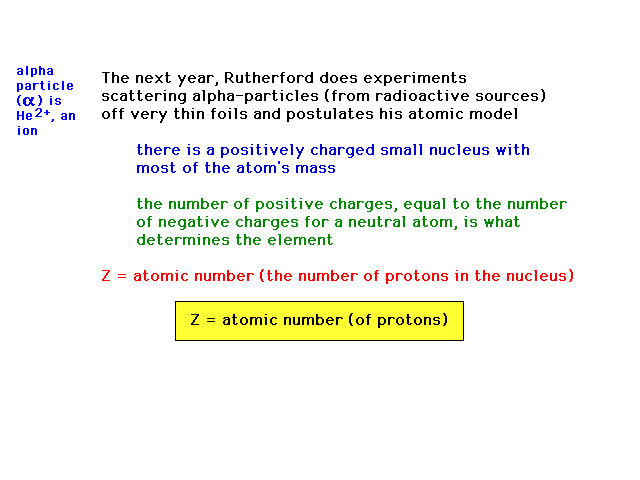
| Thompson's student Rutherford determines the
structure of an atom by studying the scattering of fast
charged particles from atoms. He finds most of the mass
and all the positive charge concentrated in a small
central core, called the nucleus. The number of units of
positive charge, Z, will determine the element (in
contrast to Dalton's postulate declaring it was
weight/mass that determined the element). The number of
electrons exactly balances the number of positive charges
for an atom, which, by definition is neutral...uncharged. |
 |
| A valuable contribution is made by the invention of
the mass spectrometer in which particles are separated
according to their mass-to-charge ratio, m/q. (H is the
strength of the magnet.) This is like Figure 2-19 in the
text. |
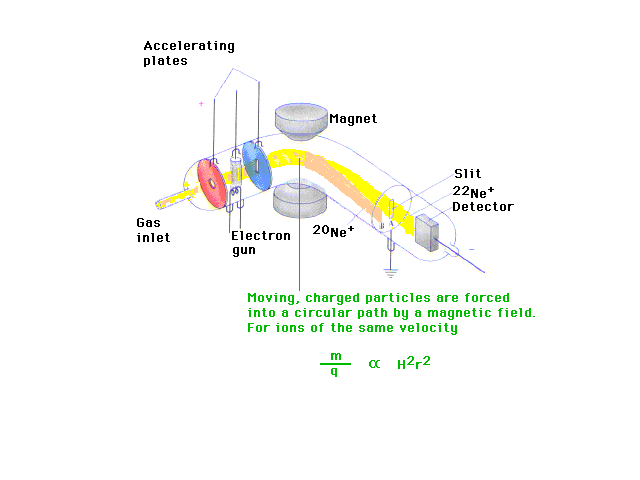 |
| Isotopes are revealed in mass measurements. They are
due to the second constituent of nuclei, the neutrons,
which are similar in mass to protons but which have no
electrostatic charge. Isotopes are indicated by mass
numbers -- integers -- which are placed as superscripts
by the symbol, usually in front of the symbol but
sometimes after it. |
 |
| The existence of isotopes is confirmed in which
distinct atomic masses of the same element (neon in this
case, as in Figure 2-20) are separated by a mass
spectrometer. |
 |
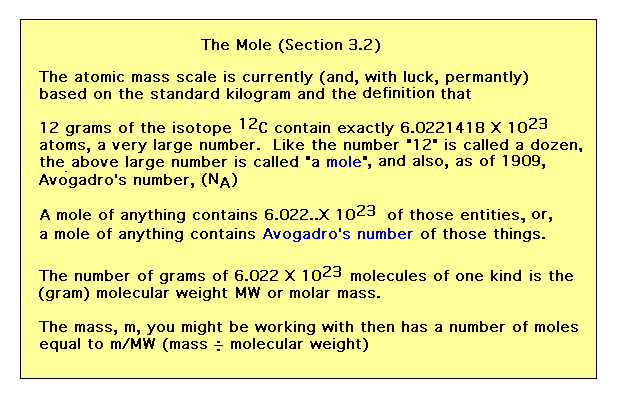
| An absolute mass scale is needed to translate our
relative atomic masses into something that can actually
be measured without having to use hydrogen gas, for
example. The benchmark has changed over the years and has
currently settled on using the isotope C-12 as the link.
The term "mole" enters our vocabulary and is an
extremely useful shorthand word representing a very large
number...Avogadro's number. |
 |
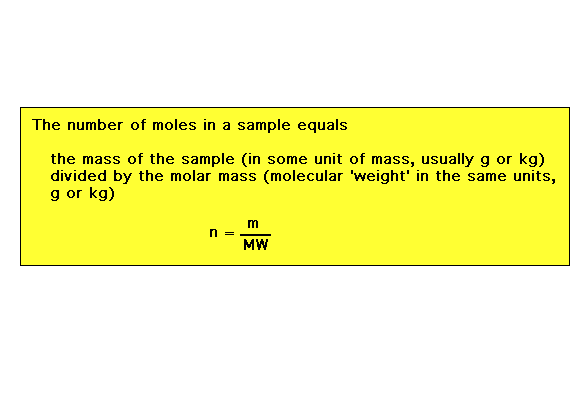
| In fewer words, mass of a substance and number of
moles of a substances are two different ways of talking
about amounts. Stoichiometry involves amounts. |
 |


