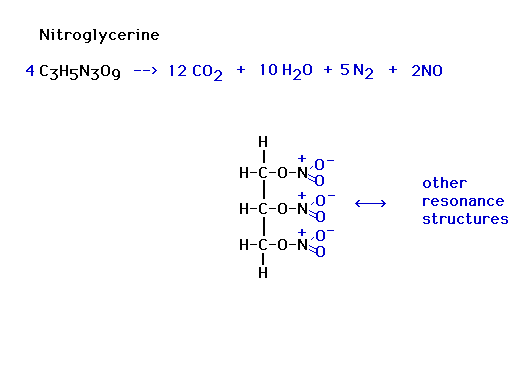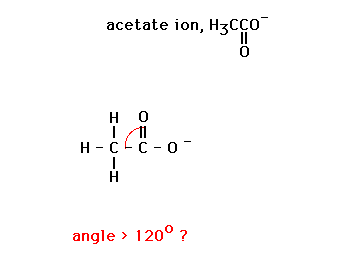| Lecture
#18 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Review for Exam II |
| We will use VSEPR to determine bond angles in a
collection of different illustrations some of which are
shown here. |
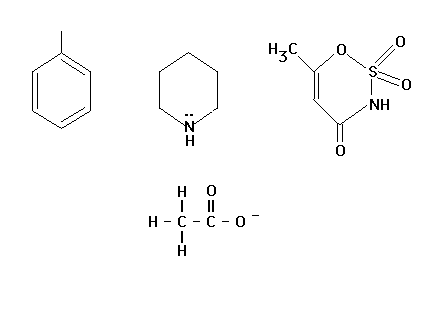 |
| Several lectures ago, we asked about the remaining
structural isomers of C4H8. The
skeletal structures are shown. One is four carbons in a
row. (It doesn't matter whether this is drawn straight,
crooked, or even L-shaped.) The other frame is branched. |
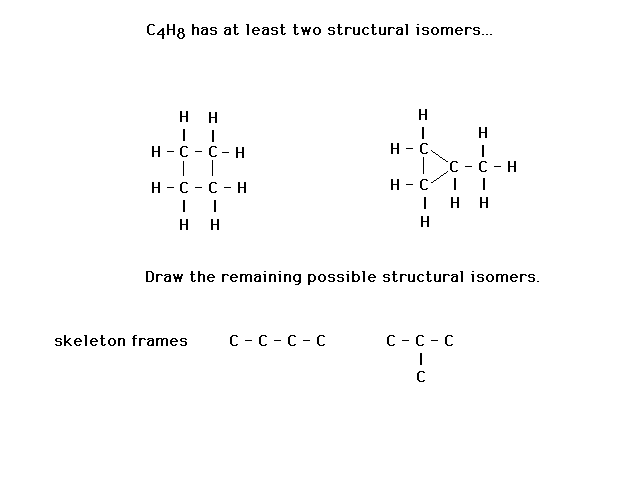 |
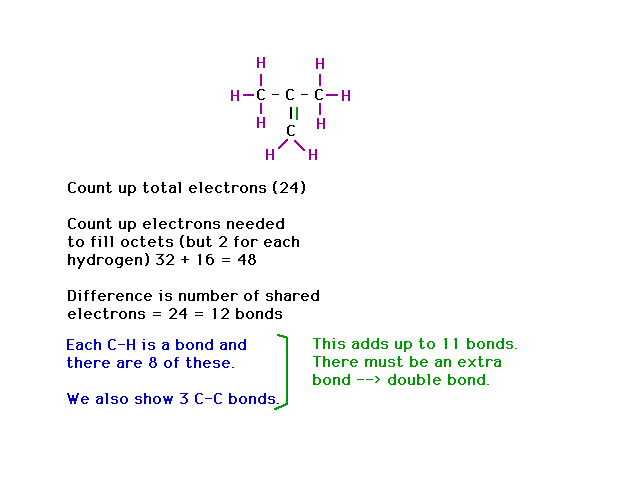
| The text gives a recipe for figuring out Lewis
structures. However, it is only useful if you know where
the atoms are. Furthermore, it does not take into account
the possibility of expanded octets (which, however, are
not relevant to this example). We follow the recipe to
note that a double bond must be present. That allows us
to place the hydrogens correctly. |
 |
| Similarly, for the non-branched skeleton, there must
be a double bond. But it can be in the center or at the
end. That gives us two more structural isomers. |
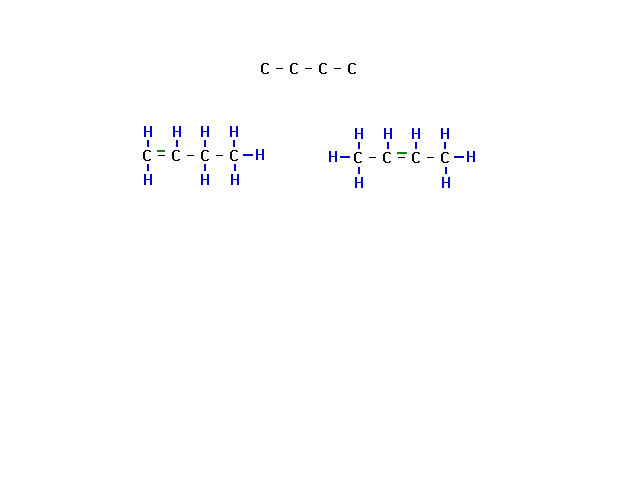 |
| Examples: (Reaction Heat...think of it as balancing a
financial account, reactants require energy to break up,
products supply energy to do this, what energy makes this
all balance?) Energy released or energy input to the
reactants? |
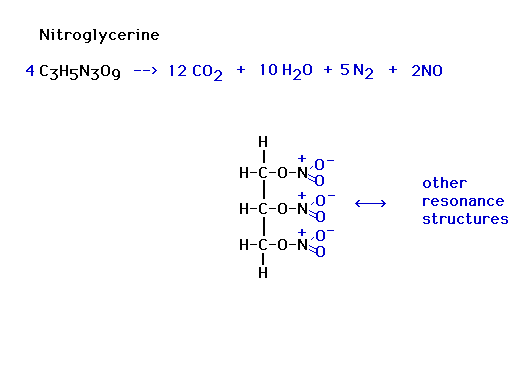 |
| Careful! |
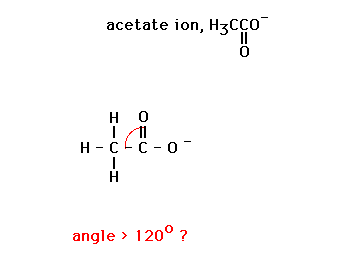 |
| What's expected for geometrical isomers for these
two? |
 |
| There is more than one geometrical isomer that can be
constructed at first. |
 |