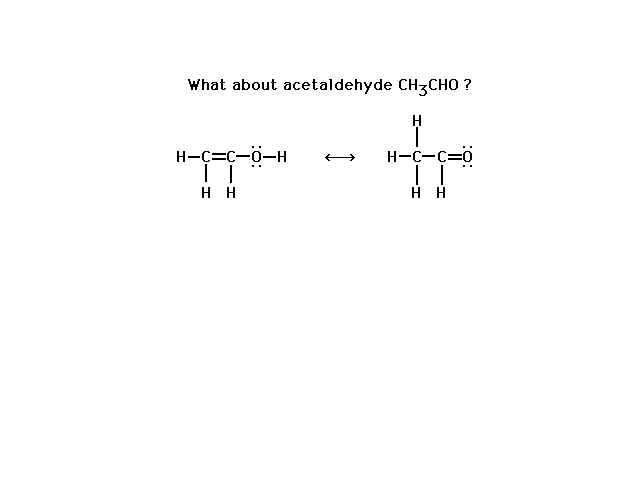| Lecture
#12 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Molecular Structure
Exceptions to the Octet Rule
Odd electron numbers
Expanded octets (hypervalency)
Examples
Resonance
Equivalent preferred contributors
Bond order
|
| Another exception to the octet rule occurs in
molecules that have an odd number of electrons. You can't
get an even number (eight) working with an odd number. |
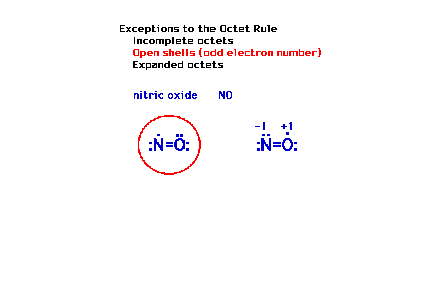 |
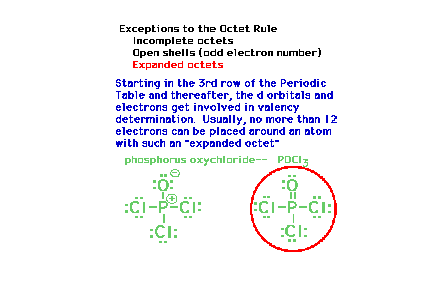
| There is also the possibility of having more
than eight electrons around an atom once the d-levels
become accessible. This happens in the third row of the
periodic table, most significantly for elements with Z
greater than 13. (The term "hypervalency"
is sometimes used to describe this effect.) |
 |
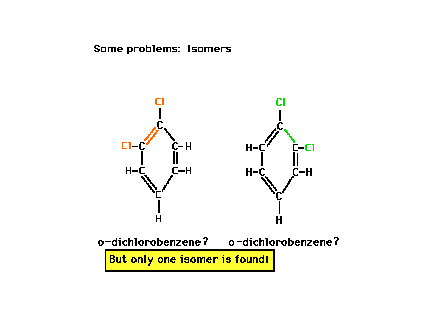
| Benzene is the cyclic hydrocarbon C6H6.
The two Lewis structures drawn here look to be structural
isomers. On the left, the chlorines are adjacent to a
double bond. On the right, they are adjacent to a single
C-C bond. Yet only one isomer exists. Why? The answer
lies in the phenomenon called "resonance." |
 |
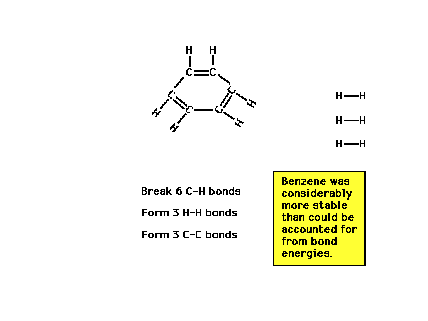
| We could estimate the energy involved in the reaction
shown by looking at bonds broken and bonds formed during
the electrons' rearrangements. (These energies are called
reaction heats and will be the subject of
further discussion in the next lecture.) |
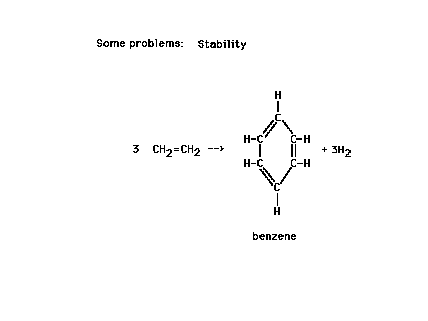 |
| But the value obtained differs significantly fromwhat
is actually measured. Why? ..."Resonance!" |
 |
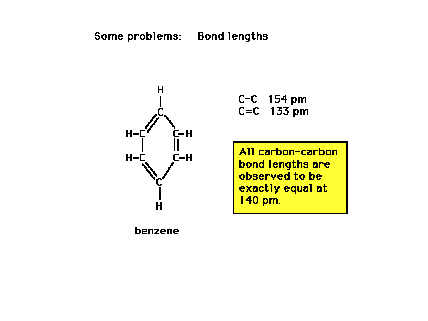
Despite a Lewis structure indicating alternating
single and double bonds...alternating long and short
carbon-carbon separation distances, all C-C bond lengths
are identical. Why?..."Resonance!"
What, then, is resonance? |
 |
| Benzene is the cyclic hydrocarbon C6H6.
The two Lewis structures drawn here look to be structural
isomers. On the left, the chlorines are adjacent to a
double bond. On the right, they are adjacent to a single
C-C bond. Yet only one isomer exists. Why? The answer
lies in the phenomenon called "resonance." |
 |
| We could estimate the energy involved in the reaction
shown by looking at bonds broken and bonds formed during
the electrons' rearrangements. (These energies are called
reaction heats and will be the subject of
further discussion in the next lecture.) |
 |
| But the value obtained differs significantly fromwhat
is actually measured. Why? ..."Resonance!" |
 |
Despite a Lewis structure indicating alternating
single and double bonds...alternating long and short
carbon-carbon separation distances, all C-C bond lengths
are identical. Why?..."Resonance!"
What, then, is resonance? |
 |
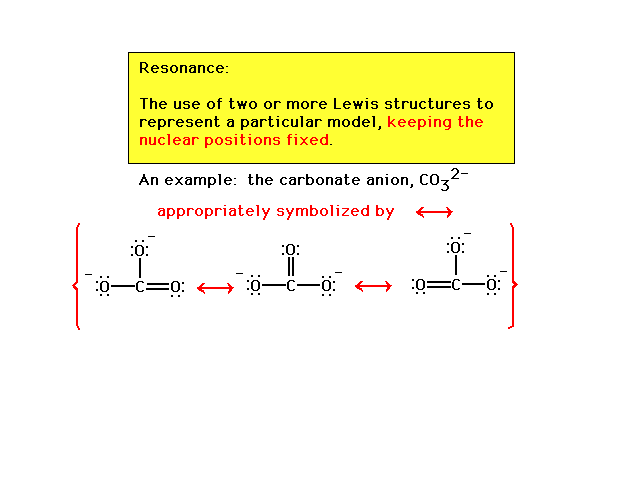
| Resonance is condition associated with two or more
arrangements of valence electrons. We will be concerned
with those that give equivalently preferred
Lewis structures. |
 |
| First illustration of resonance among equivalent
Lewis structures. The Lewis structure is represented
by this mixture; not by the three arrangements, but by a single
arrangement of electrons too complicated to be drawn
on one picture, and so we resort to symbolizing that one
picture as a superposition of three pictures we can
draw. |
 |
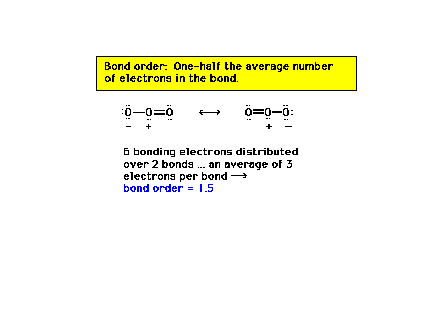
| Previously, we had single, double, and triple bonds
corresponding to bond orders of one, two and
three, respectively. But with resonance structures, we
need a modification of our definition of bond order to
accommodate intermediate possibilities. Here we
illustrate resonance structures and bond order using
ozone (O3) as an example. |
 |
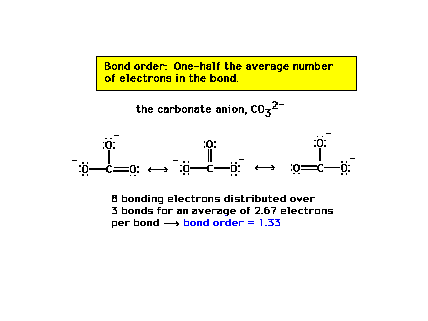
| The carbon-oxygen bond order in the carbonate ion is
1.33 for each of the three CO bonds. |
 |
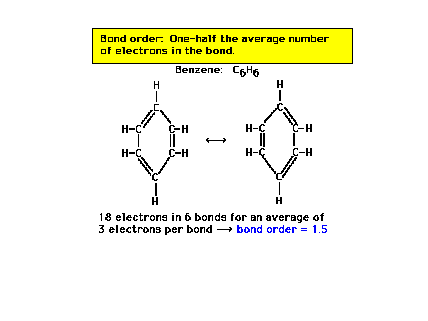
| For benzene, the carbon-carbon bond order is
exactly halfway between a pure single bond and a pure
double bond. |
 |
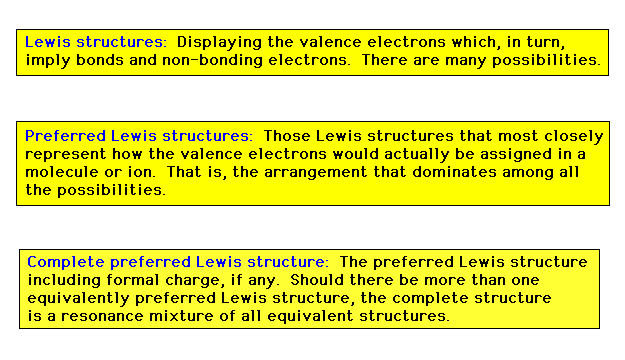
| Any reference to a complete, preferred Lewis
structure will imply that formal charges must be
indicated where necessary and all resonance contributions
of equivalently preferred structures are needed as well. |
 |
| The nitrate ion preferred structure. |
 |
| Illustrating what is meant by equivalent structures
using an example where structures are not
equivalent. |
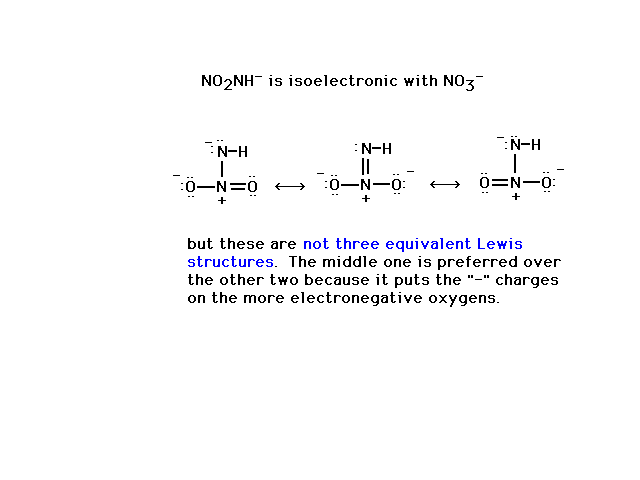 |
| This is not a pair of resonance
structures for CH3CHO. Check the rule for resonance
structures several slides back. |
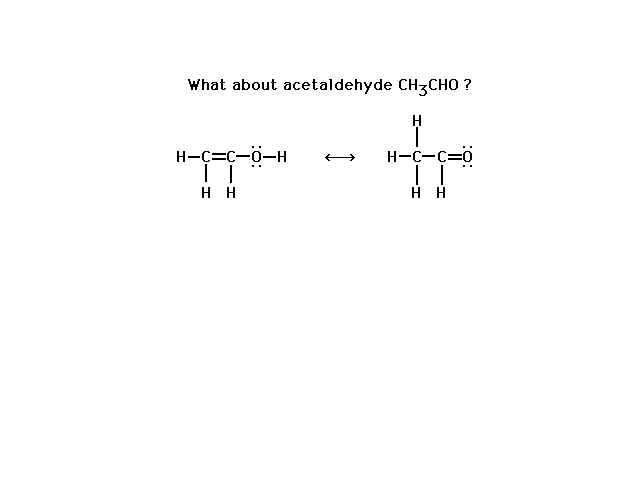 |