| Lecture
#13 |
| |
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
Molecular Structure
Resonance
Equivalent preferred contributors
Bond order
Line Structures
Reaction Heats
|
| A sample question about Lewis structures and bond
orders for some oxide ions of astatine (Z=85). |
 |
| Introducing the shorthand symbolism of "line
structures". This is not in the text. |
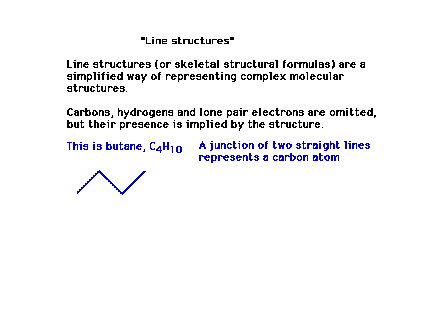 |
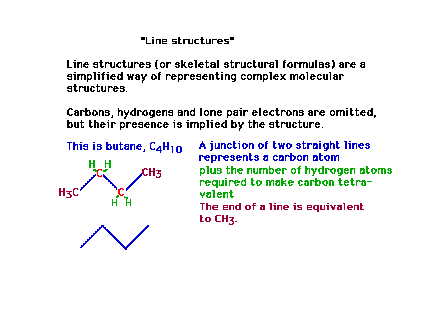
| Line structures are a useful and very common
way of presenting Lewis structures. They are essentially
geometric abbreviations. |
 |
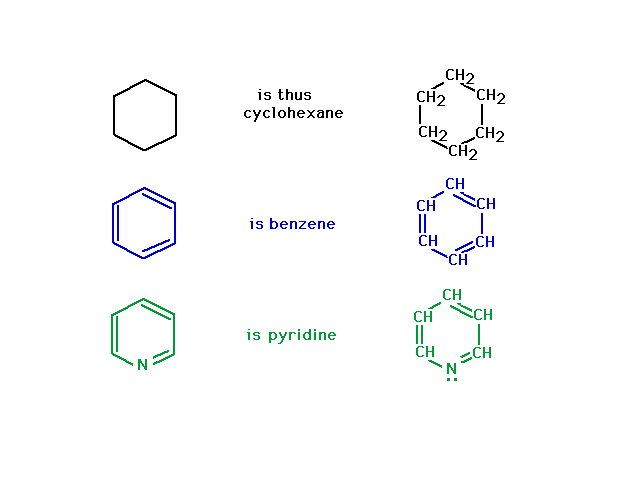
| Here are two illustrations of line structures
that look similar, but aren't quite the same. |
 |
| An application: We'll diverge
slightly and discuss bond energies and one way in which
they can be applied, the energy released (reaction
heat) in the combustion of octane. Here we get the
balanced equation for the complete combustion reaction.
(Reaction heats are not covered in the
text.) |
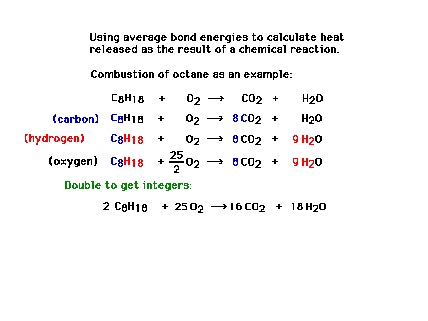 |
| In order to take a look at the energy change
involved, we look at the "structures" of the
various reaction components. |
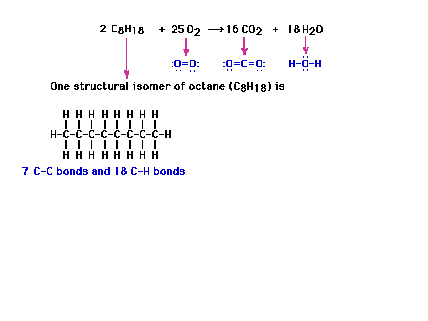 |
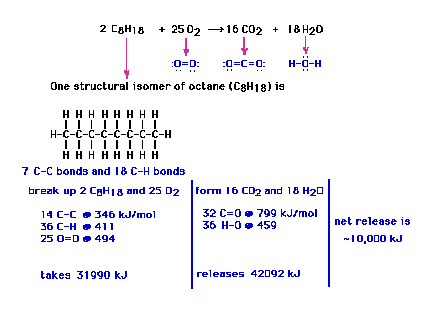
| We can then calculate the net energy change when
electrons re-arrange their "allegiance", that
is, some bonds break and others form. The various bond
energies can be obtained from data bases (tables in the
text, for example) |
 |