| Lecture
#1 |
|
| CURMUDGEON
GENERAL'S WARNING. These "slides"
represent highlights from lecture and are neither
complete nor meant to replace lecture. It is
advised not to use
these as a reliable means to replace missed
lecture material. Do so at risk to healthy
academic performance in 09-105. |
|
| Lecture Outline |
History
The first 2000 years
Stoichiometry Laws
Conservation of Mass
Law of Definite Proportions
Law of Multiple Proportions
Law of Combining Volumes
Avogadro's Law
|
| A quick history of the development of chemistry as a
science starts in ~-400 BC. |
 |
| Measurements led to one of the important early laws
of chemistry, the Law of Conservation of Mass. The text,
p. 17, expresses this as in every chemical operation
"mass is neither created nor destroyed." |
 |
| Some chemical discoveries in the early 1800s. |
 |
| Experimental measurements led to the second important
early observation, the Law of Definite Proportions. The
text, p. 17, expresses this Law as "a given
compound always contains exactly the same proportion of
elements by mass." |
 |
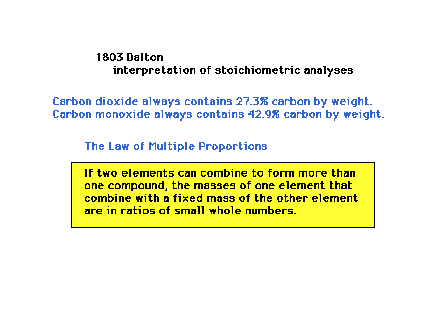
| John Dalton observed a pattern in results of chemical
analysis on compounds (substances made from two or more
elements) made from the same elements. For example,
carbon and oxygen can produce carbon dioxide and carbon
monoxide. From analyses of their composition by weight
and similar analyses on other sets of compounds, he
produced the "Law of Multiple Proportions". On
p. 18, it reads as "When two elements form a
series of compounds, the rations of the masses of the
second element that combine with 1 gram of the first
element can always be reduced to small whole numbers." |
 |
| This is a numerical illustration (not done during
lecture) of Dalton's Law of Multiple Proportions showing
how "small whole numbers" emerge. |
 |
| John Dalton and his 1803 Theory of Atomic Theory of
Matter. The last postulate is referred to as the
"Postulate of Simplicity." |
 |
| Dalton's Theory has some crucial faults. |
 |
| The French scientist, Gay-Lussac, studied
reactions of gases all at the same temperature and
pressure and noted a pattern involving the gas volumes. |
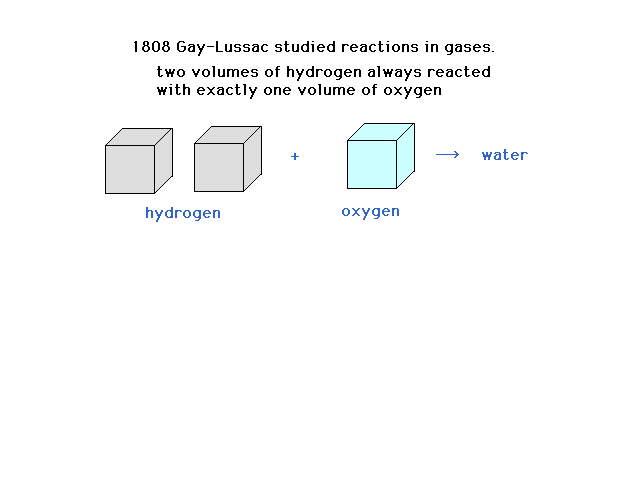 |
| Gay-Lussac's study of combining volumes of gases
provides a clue |
 |
| Avogadro accepts the significance of Gay-Lussac's
experiments and hypothesizes some significant ideas. |
 |
