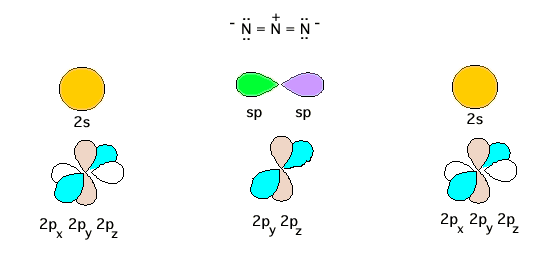
Problem 16.23 is correctly based on the same electronic structure as that for CO2 given in lecture. However, the text authors mistakenly thought through a different construction which, you will recognize, is incorrect(!). Nevertheless, I thought it might prove beneficial to show you what they were thinking. To begin, here's the Lewis structure for N3- and the atomic orbitals from which the molecular orbitals should evolve. Note immediately that there is no resonance evident in this Lewis structure. Yet the textbook will invoke the need for resonance (incorrectly).
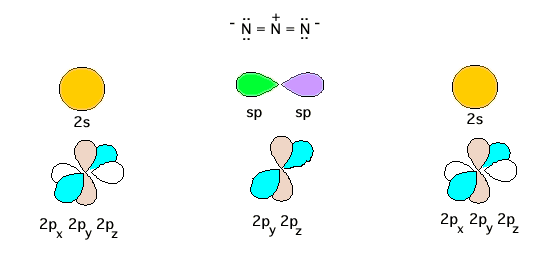
The central N has steric number 2 and therefore a pair of sp hybrids, as shown above, and also two unhybridized 2p atomic orbitals, as also shown. The authors incorrectly leave the end nitrogens completely unhybridized with atomic 2s, 2px, 2py, and 2pz orbitals as shown. First, each central N sp hybrid will form a sigma bond with the pure 2p oriented on the bond axis. This uses two pair of valence electrons
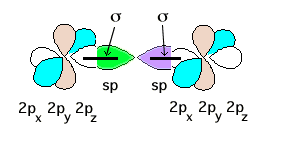
On the end N's, each atomic 2s orbital (no longer shown) acquires a lone pair of electrons. There are now 4 pair of valence electrons remaining. The central N pure 2p orbitals overlap with both pair of 2p orbitals on both end nitrogens to give delocalized orbitals encompassing the triatomic system. One such overlap (brown 2ps) is schematically shown below.
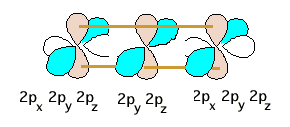
There are three pair of 2p orbitals involved: a total of six atomic orbitals which give rise to six molecular orbitals into which the remaining 4 pair of electrons get dumped. The energy diagram below is the same as that gotten for ozone in Friday's lecture, where one collection of three 2p orbitals were used, except here we double up because we have the second identical set (the blue 2ps above) perpendicular to the first (brown) set. Now the follow-up questions about N3 and N3+ make more sense, but are based on an incorrect orbital picture.