CARNEGIE MELLON UNIVERSITY
09-105 Introduction to Modern Chemistry
Quiz #4
October 1, 2001
Name KEY (2 pts each)
Section /Instructor
| H | He | ||||||||||||||||
| Li | Be | B | C | N | O | F | Ne | ||||||||||
| Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr |
| Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe |
| Cs | Ba | La* | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn |
| Fr | Ra | Ac* | Rf | Db | Sg | Bh | Hs | Mt | 110 | 111 | 112 | 114 |
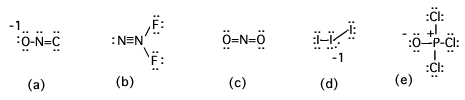
Does the structure shown for (a) represent a correct, complete, preferred Lewis structure for a molecule with the formula CNO-? (Yes or no)
No. As shown, the C would need a formal charge of 2- making the species CNO3-.
Does the structure shown for (b) represent a correct, complete, preferred Lewis structure for a molecule with the formula N2F2? (Yes or no)
No. The central N is shown with an expanded octet, which it is not capable of having.
Does the structure shown for (c) represent a correct, complete, preferred Lewis structure for a molecule with the formula NO2+ in which N is central? (Yes or no)
No. It's incomplete. The N should show the formal charge 1+.
Does the structure shown for (d) represent a correct, complete, preferred Lewis structure for a molecule with the formula I3-? (Yes or no)
Yes.
Does the structure shown for (e) represent a correct, complete, preferred Lewis structure for a molecule with the formula POCl3 in which P is central? (Yes or no)
No. If P is given an expanded octet, the O/P bond can become a double bond eliminating the formal charges on the structure. That is preferred.