CARNEGIE MELLON UNIVERSITY
09-105 Introduction to Modern Chemistry
Quiz #8
November 19, 1998
Name
Cobalt (III) forms octahedral complexes with coordination number 6. Sketch the structures of all the complex ion's isomers that would be found associated with the neutral compound Co(H2O)Cl2(NH3)3F using the frames below. More than enough might have been provided. Use the back if more are needed. (You need not have done all, an unreasonable task it turns out.)
Geometrical Isomers
 |
For each of the structural isomers below, there are possibilities of a variety of geometrical isomers. Some examples involving [Co(H2O)Cl2(NH3)3]+ , for example, are where the two Cl's are "trans" vs when they are "cis" to each other; in the latter case when the water is trans to one Cl vs when it is cis to both. You need not present too many of the variety of possibilities to get full credit. (There are too many geometrical isomers here.) (1 point each up to a total of 5 points) |
Structural Isomers
 |
The
structural isomers that are possible are any arrangements
of the following types of species: [Co(H2O)Cl2(NH3)3]+ F- |
Optical isomers
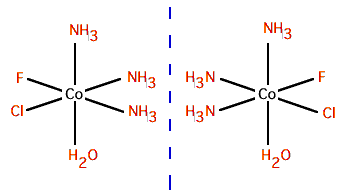 |
There
is a pair of optical isomers. (2 points) |