Department of Chemistry
CARNEGIE MELLON UNIVERSITY
09-105 Introduction to Modern Chemistry
Quiz #6 Name
Closed book, 10 minutes October 29, 1998
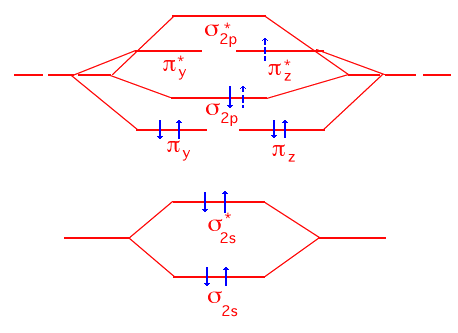
Complete the molecular orbital energy diagram (correlation diagram) below so that the ground state electron configuration for N2 is illustrated. When molecular nitrogen absorbs ultraviolet light of the appropriate wavelength, an excited electronic state is produced because the highest energy electron in N2 moves up to the next available electronic state. Show the molecular orbital energy diagram for this excited state and indicate whether its nitrogen-nitrogen bond length should be longer, shorter or the same as it was in the N2 ground electronic state.
 |
The original nitrogen molecule has a bond order of 3 because there are a total of six net bonding electrons. When the highest electron (from the s2p state) is promoted to the next available state, the latter is an antibonding state as shown with the dashed spin arrows. The net bond order drops to 2 from which you can legitimately infer that the NN bond length would become longer than in the ground state. |
 |
Make certain you have answered all parts.