| The sp
- py,z Sequence |
|
| The standard sequence of sp
and py,z
|
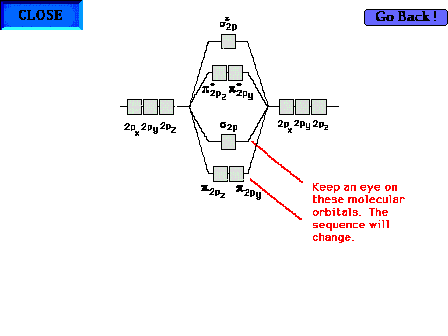 molecular orbitals. molecular orbitals. |
| The change in the sequence of bonding sp and py,z
orbitals for oxygen and fluorine. |
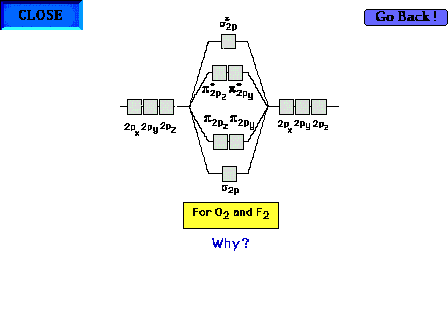 |
| Electron configurations across the first complete row
of the periodic table (lithium and up). |
 |
| The 2s atomic orbitals are present in
all of these configurations. |
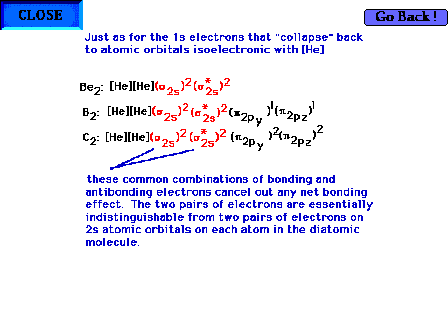 |
| The geometry of the non-bonding (1s and 2s) orbitals
in Be2 |
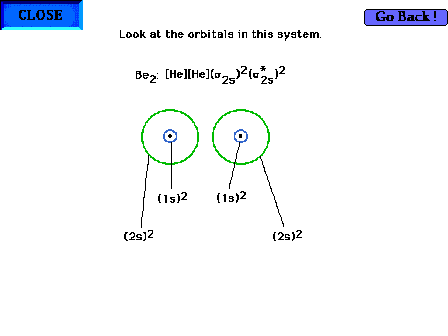 |
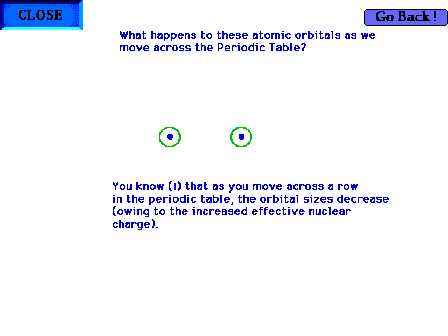
| The change in the geometry of the non-bonding (1s and
2s) orbitals as we move across the row of the periodic
table towards oxygen and fluorine. The orbitals get
smaller because the nuclear charge is increasing. |
 |
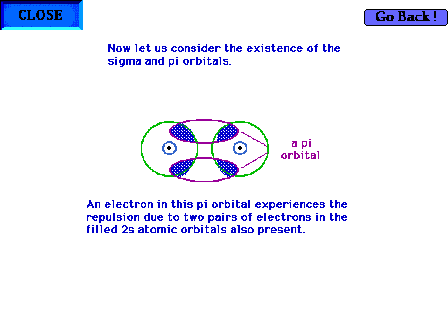
| The geometry of a pi molecular orbital in, for
example, B2. The shaded region shows where
electron-electron repulsions are particularly
unfavorable. |
 |
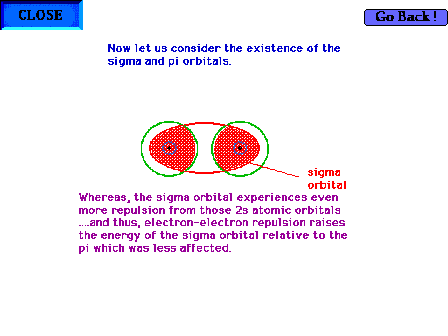
| The geometry of a sigma molecular orbital in, for
example, B2 showing that the electron-electron
repulsions, most effective in the shaded regions, are
even worse than in the case of the pi orbital's
electrons. The sigma orbital is consequently less stable
than the pi orbitals. |
 |
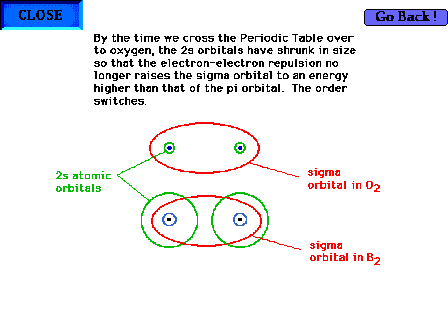
| The geometry of a sigma molecular orbital in O2
vs B2. |
 |
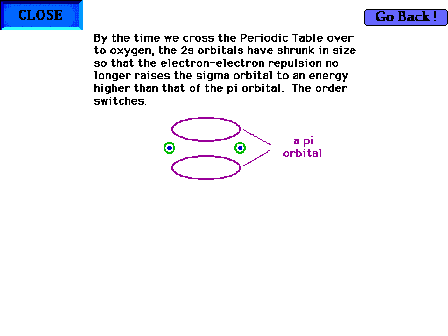
| The geometry of a pi molecular orbital in, for
example, O2. |
 |
| Return
to list of lectures. |
|