Section 1.01
Biochemistry I, Fall
Term Sept
2, 2005
Lecture
3: Acid-Base Equilibria &
Buffers
Reading in Campbell: Chapter 2.3-2.6
Key
Terms:
- Acid strength
- Equilibrium constant
- Acid-base properties of
H2O
- Acid dissociation
constant
- pH = - log[H+]
- pH = pKa + log[A-]/[HA]
- Titration: pKa
determination
- Equivalence point, Inflection point
- Calculation of degree of protonation
3.1
Acids and Bases and Acid Strength
- Acid: can donate protons
- Base: can accept protons
Example:
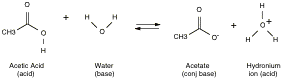
Acid
strength:
A
compound is an acid if it loses its proton, and it is a base if it takes up a
proton. For a simple acid like
X-H, acid strength depends on the strength of the X-H bond and the stability of
the charged anion X-. Weaker
bonds generally lead to greater acidity.

H2O < H2S < H2Se
HF < HCl < HBr
HF > H2O
HCl > H2S
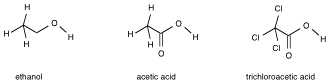 Consider the following three
compounds:
Consider the following three
compounds:
The
negative charge on the ionized acid can be further stabilized by resonance
structures that distribute the negative charge as well as by nearby electron
withdrawing groups. The nearby
presence of formal positive and negative charges also affects acidity.
3.2
General Equilibrium Reactions:
Consider
first a very simple reaction and its equilibrium features:
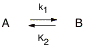
The
following rate equations can be used to describe the reaction:
![]()
![]()
At
equilibrium there is no change in the concentration of A or B, therefore:
![]()
At
equilibrium the reaction has not stopped, but rather, the rates of the forward
reaction (k1[A]) and the reverse reaction (k2[B]) are
equal. Thus:
![]()
3.3
Ionization of H2O and definition of pH:
![]()
The
equilibrium constant for the dissociation of H2O can be written:
![]()
Since
the concentration of H2O is high (55.5 M) and practically constant,
we can incorporate it into the equilibrium constant and define a dissociation
constant for H2O:
![]()
Pure H2O
is neutral. Therefore ionization
produces equal concentrations of H+ and OH-, and
![]()
There
is a reciprocal relationship between [H+] and [OH-]. In solutions that are acidic, [H+]
is high and [OH-] is low.
Conversely, in solutions that are alkaline, [H+] is low and
[OH-] is high. Note
that the ion product is always ![]() .
.
pH
Definition: pH is measured as the ![]() . The lower the
pH, the more acidic the solution.
Neutral pH is 7.0. At this
pH there are an equal number of H+ and OH- ions in
solution.
. The lower the
pH, the more acidic the solution.
Neutral pH is 7.0. At this
pH there are an equal number of H+ and OH- ions in
solution.
Relation
of [H+] to [OH-] to pH
|
pH |
|
[H+], M |
|
[OH-], M |
|
0 |
|
100 |
|
10-14 |
|
1 2 3 |
|
10-1 10-2 10-3 |
|
10-13 10-12 10-11 |
|
4 |
|
10-4 |
|
10-10 |
|
5 6 |
|
10-5 10-6 |
|
10-9 10-8 |
|
7 8 |
|
10-7 10-8 |
|
10-7 10-6 |
|
9 10 11 12 13 14 |
|
10-9 10-10 10-11 10-12 10-13 10-14 |
|
10-5 10-4 10-3 10-2 10-1 100 |
---
Hydrochloric Acid (1 M)
---
Human gastric contents
---
Tomato juice
---
Cow¹s milk
---
Human blood plasma
---
Human pancreatic juice
---
Ammonia (1 M)
---
Sodium hydroxide (1 M)
3.4
Characterization of Acid Strength using pKa.
When an
acid HA is added to H2O,
![]()
The
equilibrium constant for its dissociation is defined:
![]()
![]()
The
acidity constant, Ka, is a fundamental property of
the acid and does not depend on the pH of the solution. Stronger acids have larger Ka values since they are more
fully dissociated.
The
Henderson-Hasselbalch Equation: Since the
proton concentration is always measured in units of pH, it is useful to modify
the above equation by taking the negative log·:
![]()
which
gives rise to the Henderson-Hasselbalch equation:
|
|
The pKa is the log Ka. Therefore strong acids have small pKa values.
Monoprotic
acids (release
only one proton):
|
|
|
||||||||||||||||||||
|
|
|
|
3.5
Solving pH Problems:
The
H&H equation has predictive value.
Once you know the pKa of an acid and the pH, you can predict
[A-]/[HA]. One
particular case is routinely used in biochemistry: Given a pH and pKa
of an acid, calculate the fraction of the acid that is protonated: fHA = ([HA]/AT) and the fraction that is
deprotonated:
fA- = ([A-]/AT),
where AT is the total concentration of acid: AT = [HA] +
[A-].
Defining
R = [A-]/[HA]
pH
= pKa + log([A-]/[HA])
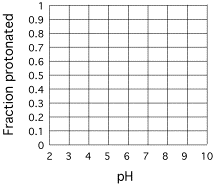 pH
= pKa + log(R)
pH
= pKa + log(R)
pH pKa = log(R)
10(pH-pKa)
= R
Once R
is found, the fraction protonated and
deprotonated
is obtained as follows:
[A-]/[HA]
= R
[A-]
= [HA]R
[AT]
= [A-] + [HA]
[AT]
= [HA](1+R)
![]()
![]()
Using
the ionization of the side chain of the amino acid Histidine as an example (pKa
= 6.0)
|
pH |
(i) R |
FHA |
|
4 5 |
R =
10(4-6) = 10-2 R =
10(5-6) = 10-1 |
FHA
= 1/(1+0.01)= 0.99 FHA
= 1/(1+0.1) = 0.91 |
|
6 |
R =
10(6-6) = 100 |
FHA
= 1/(1+1) = 0.5 |
|
7 |
R =
10(7-6) = 101 |
FHA
= 1/(1+10) = 0.091 |
|
8 |
R =
10(8-6) = 101 |
FHA
= 1/(1+100) = 0.01 |
There
are four general statements that are useful to remember:
1.
When
the pH = pKa, [HA] = [A-].
2.
When
the pH is lower than the pKa, [HA]
> [A-].
3.
When
the pH is higher than the pKa, [HA]
< [A-].
4.
A
pH change of 1 leads to a 10 fold change in the ratio of [A-]/[HA].
3.6
Titration Curves
Ka values are usually measured by direct
experiment, usually with a pH titration.
Known amounts of a strong base (NaOH) are added to a solution of a weak
acid and the pH is measured as the amount of NaOH is added. As the base is added it removes the
proton from the acid, as well as increasing the pH.
Inflection
point (pH =
pKa): You can prove from the Henderson-Hasselbalch equation that
the smallest change in pH due to addition of base occurs when the pH = pKa; at this inflection point, the pH of the
solution is the pKa of the acid.
Equivalents: moles of base/moles of acid, the x-axis for titrations. Varies from 0 to 1 for monoprotic
acids.
Equivalence
point: Complete deprotonation of the weak acid
occurs when the molar amount of base is equal to, or equivalent to, the molar
amount of weak acid. This point in
the titration is referred to as the equivalence point.
Example
titration curve:
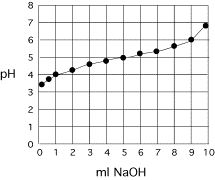 ml NaOH measured
pH
ml NaOH measured
pH
0.25
3.20
0.5
3.80
1
4.07
2
4.44
3
4.62
4
4.87
5
4.96
6
5.17
7
5.33
8
5.64
9
5.96
10
7.00
Facts: Concentration of NaOH =
1 M
Volume
of solution that is titrated = 100 ml
Possible
acids (from Campbell table 2.6)
Pyruvate pKa = 2.5
Acetic
Acid pKa = 4.8
Tris pKa = 8.3
Questions:
1.
What
is the pKa of the acid?
2.
Which
acid is it?
3.
Give
the x-axis scale in equivalents.