[For this illustration we will use concentration units of "balls/Box" (b/B) instead of M (mol/liter).]
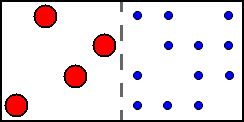 Starting concentrations (b/B):
Starting concentrations (b/B):
Left cell: [ML] = 0; [L] = 0; [M] = 4.
Right cell: [ML] = 0; [L] = 12; [M] = 0.
When equilibrium is reached, the concentration of free ligand will be the same in both cells. However, because the protein can bind the ligand, the concentration of total ligand will be higher in the left cell.
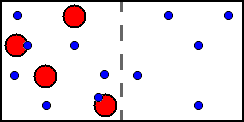 Equilibrium concentrations (b/B):
Equilibrium concentrations (b/B):
Left cell: [ML] = 2; [L] = 5; [M] = 2.
Right cell: [ML] = 0; [L] = 5; [M] = 0.
The above illustrations are static "snapshots" of the molecules during the experiment.
Open a window that simulates the Molecular Motion at Binding Equilibrium.
(The animation requires the Shockwave plug-in.)
With the above results we can calculate the Kd for this cartoon binding reaction:
Kd = [M][L]/[ML] = (2 b/B)¥(5 b/B)/(2 b/B) = 5 b/B.
Note the following features of this binding equilibrium:
[L] = 5 b/B in both cells.
[L]total = 7 b/B in the left cell.
[L]total - [L] = 2 b/B = [ML] in the left cell.
Thus, the difference in [L]total between the two cells is the measurement of [ML] that we need to add one datum to a Scatchard plot. A Scatchard plot consists of equilibrium dialysis measurements done at several starting concentrations of ligand. (See the Ligand Binding page for an example of the calculations and graphing procedures.)
For example, with the Kd calculated above, what do you predict for the equilibrium concentrations of all species if the starting concentration of ligand had been 33 b/B? or 5 b/B?